Material properties
The comparison of alkanolamide and silane coupling agent
on the properties of silica-
fi
lled natural rubber (SMR-L)
compounds
Indra Surya
a, H. Ismail
b,*, A.R. Azura
baDepartment of Chemical Engineering, Engineering Faculty, University of Sumatera Utara, Medan, 20155, Sumatera Utara, Indonesia bSchool of Materials and Mineral Resources Engineering, Universiti Sains Malaysia, Engineering Campus, Nibong Tebal, 14300, Penang,
Malaysia
a r t i c l e
i n f o
Article history: Received 20 May 2014 Accepted 6 August 2014 Available online 27 August 2014
Keywords: Alkanolamide Reinforcing efficiency APTES
Silica-reinforcement Plasticiser
a b s t r a c t
Alkanolamide (ALK) and Aminopropyltriethoxy Silane (APTES) wereincorporated separately into silica-filled SMR-L compounds at 1.0, 3.0, 5.0 and 7.0 phr. It was found thatcompounds withboth ALK and APTES exhibited cure enhancement, betterfiller dispersion and greater rubber-filler interaction. Both additivesalso produced modulus and tensile enhancements in thesilica-filled SMR-L compounds, especially up to a 5.0 phr loading. At a similar loading, ALK exhibited higher reinforcing efficiency of silica than APTES.
©2014 Published by Elsevier Ltd.
1. Introduction
The mechanical properties such as tensile modulus, tensile strength, tear and abrasion resistances of a rubber vulcanisate are enhanced as a result of the incorporation of reinforcingfiller into the rubber compound. Carbon black and silica are the best known reinforcingfillers, and have been widely utilised in the rubber industry. Each type of reinforcing filler produces useful mechanical properties due to their specific surface chemistry. Carbon black and silica have apparently a dissimilar surface chemistry. The surface of carbon black is saturated with hydrocarbon functional groups that react with sulphur during vulcani-sation. They form sulphur bonds that link the rubber chains, and also tie the carbon black to the rubber[1e2]. In
marked contrast to the hydrocarbon functionality of carbon black, silica does not react with sulphur. The surface of silica is highly polar and hydrophilic due to the presence of
numerous silanol groups. The silanol groups are relatively incompatible with hydrocarbon rubbers, such as natural rubber and styrene butadiene rubber, therefore coupling bonds are not formed. On the other hand, the silica parti-cles have a strong tendency to interact with each other to form aggregates. Since the silica-hydrocarbon rubber interaction is weaker than the silica-silica interaction, the results are the formation of large agglomerates, poor dispersion of silica and lower reinforcing efficiency.
Modulus is a well-recognised criterion of filler rein-forcement[3]. Due to the nature of its surface chemistry, silica has a low level of surface activity for rubber bonding, resulting in small amounts of effectively immobilised rub-ber. Therefore, silica reinforced rubbers have lower modulus than carbon black reinforced rubbers.
In order to overcome the deficiencies of silica, coupling agents are used for the reinforcement of hydrocarbon rubbers. The most efficient and presently known coupling agent is organosilane[4]. Organosilanes are reactive addi-tives. They are utilised to improve the silica-rubber inter-action of silica-filled rubbers and, consequently, enhance *Corresponding author.
E-mail addresses:ihanafi@usm.my,profhanafi@gmail.com(H. Ismail).
Contents lists available atScienceDirect
Polymer Testing
j o u r n a l h o m e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / p o l y t e s t
http://dx.doi.org/10.1016/j.polymertesting.2014.08.007
0142-9418/©2014 Published by Elsevier Ltd.
the reinforcing efficiency[5e6]. The organosilanes modify
the surface of silica[7e8]. The modified silica provides a
chemically active surface that can participate in vulcani-sation; providing coupling bonds between organosilane and both silica and rubber phases. There is much evidence confirming the existence of such bonds [9e10]. The
coupling bonds were noted to mark improvements in the mechanical properties of the rubber-filled vulcanisates [11e16].
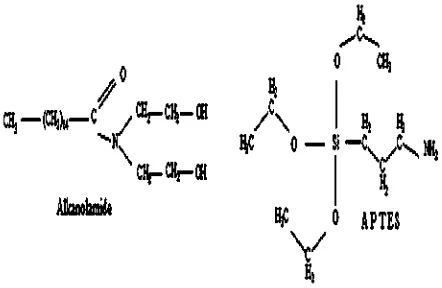
In our previous work[17], the preparation and appli-cation of Alkanolamide (ALK) in silica-filled natural rubber compounds were reported. The incorporation of ALK within silica-filled natural rubber compounds gave enhanced mechanical properties viz. tensile strength, ten-sile modulus and hardness. The enhancement of these properties was attributed to the improvement of silica dispersion in the rubber compounds, and higher crosslink density that stemmed from the incorporation of ALK. The results also indicated that ALK may function as an accel-erator and a plasticiser. In this study, the properties of silica-filled natural rubber compounds with ALK were compared with those of silica-filled natural rubber com-pounds with Aminopropyltriethoxy Silane (APTES). APTES is a type of organosilane. Similar to ALK, APTES also con-tains amine within its molecule, and its molecular structure is shown inFig. 1.
2. Experimental
2.1. Materials
Natural rubber grade SMR-L was obtained from Guthrie (M) Sdn. Bhd., Seremban, Malaysia. Other compounding ingredients such as sulphur, zinc oxide, stearic acid, N-isopropyl-N'-phenyl-p-phenylenediamine (IPPD), benzo-thiazolyl disulfide (MBTS) and precipitated silica (grade VulcasileS) were supplied by Bayer Co. (M) Sdn. Bhd., Petaling Jaya, Selangor, Malaysia. The APTES (C9H23NO3Si) was supplied by Sigma-Aldrich. The ALK was synthesised in our laboratory using Refined Bleached Deodorized Palm Stearin (RBDPS) and diethanolamine. The reaction pro-cedures and molecular characterisations of the ALK were given in our previous report[17].
2.2. Compounding
A semi-efficient vulcanisation system was applied for the compounding. The compounding procedure was per-formed on a two-roll mill (Model XK-160). Table 1 dis-played the compound designation and formulation of silica-filled SMR-L compounds with ALK and APTES.
2.3. Cure characteristics
The cure characteristics of the silica-filled SMR-L com-pounds were obtained using a Monsanto Moving Die Rheometer (MDR 2000), which was employed to deter-mine the scorch time (ts2), cure time (t90) and torque dif-ference (MHdML), according to ISO 3417. Samples of the respective compounds were tested at 150C. The com-pounds were subsequently compression-moulded using a stainless steel mould at 150C, with a pressure of 10 MPa, based on respective curing times.
2.4. Tensile, hardness and resilience properties
Dumbbell-shaped samples were cut from the moulded sheets. Tensile tests were performed at a cross-head speed of 500mm/min using an Instron 3366 universal tensile machine, according to ISO 37. The tensile strength (TS), stress at 100% elongation (M100), stress at 300% elongation (M300) and elongation at break (EB) were determined. The hardness of the samples was measured according to ISO 7691-I, using a Shore A type manual durometer. The resil-ience was studied by utilising a Wallace Dunlop Trips-ometer, according to BS 903 Part A8. The rebound resilience was calculated according to Equation(1).
% Resilience¼ ½ð1 cosq2Þ=ð1 cosq1Þ 100 (1)
where
q
1is the initial angle of displacement (45), andq
2is
the maximum rebound angle.
2.5. Scanning electron microscopy (SEM)
The tensile fractured surfaces of the natural rubber compounds were examined by using a Zeiss Supra-35VP scanning electron microscope (SEM) to obtain informa-tion regarding the filler dispersion, and to detect the possible presence of micro-defects. The fractured pieces were coated with a layer of gold to eliminate electrostatic charge build-up during examination.
2.6. Measurement of rubberefiller interaction
The rubber-filler interaction was determined by swelling the cured silica-filled SMR-L compounds in toluene, according to ISO 1817. Test pieces with dimensions of 30mm5mm2mm were prepared from the moulded sheets. The initial weights were recorded prior to testing. The test pieces were then immersed in toluene and conditioned at room temperature in a dark environment for 72 hours. After the conditioning period, the weights of the swollen test pieces were recorded. The swollen test pieces were then dried in an oven at 70C for 15 minutes
Fig. 1.Molecular structure of Alkanolamide and APTES.
and allowed to cool at room temperature for another 15 minutes before thefinal weights were recorded. The Lorenz and Park's equation[18e20]was applied in this study. The
swelling index was calculated according to Equation(2).
Qf=Qg¼ae zþb (2)
where, the subscripts f and g referred tofilled and gum vulcanisates, respectively; z was the ratio by weight offiller to hydrocarbon rubber in the vulcanisate; while a and b were constants. The higher the Qf/Qg value, the weaker the rubber-filler interaction became.
In this study, the weight of the toluene uptake per gram of hydrocarbon rubber (Q) was calculated based on Equa-tion(3).
Q¼ ½Swollen Dried weight=½Initial weight
100=Formula weight (3)
3. Results and discussion
3.1. Effects of ALK and APTES on cure characteristics of silica-filled SMR-L compounds
The effects of ALK and APTES on the scorch and cure times of silica-filled SMR-L compounds are shown inFig. 2. It is well-known that the addition of a silicafiller into a SMR-L compound (defined as the control compound) cau-ses cure retardation. Due to its high polarity, silica inter-acted with zinc oxide during curing and formed silica bound zinc, which was unable to activate the accelerator. Consequently, zinc activity was reduced and the curing was retarded[2,21]. Compared to the control compound, the addition of ALK and APTES decreased the cure and scorch times of silica-filled SMR-L compounds. Both additives may be considered as co-curing agents since the polar parts of these additives reacted with the silanol groups of silica to transform the hydrophilic silica into hydrophobic silica; which interacted relatively less with zinc oxide. In this manner, the performance of zinc oxide in activating the accelerator was maximised. It was seen that the higher the loading of these additives, the lower the scorch and cure times. This was attributed to the amine-content of both additives. As presented inFig. 1, both chemicals contained amine within their molecules. Amine, being one of the
accelerator activators, is an alkaline substance that in-creases the cure rate[22]. Amine may also be utilised to overcome cure retardation problems in silica reinforcement [2,23].
The scorch and cure times of ALK were longer than those of APTES. This was attributed to the concentration of amine in each of the additive molecules. From the molec-ular structures of the additives, as presented inFig. 1, it was seen that the mass fraction of amine in ALK was lower than that of APTES. Therefore, at a similar loading, ALK provided a lower concentration of amine than APTES.
Table 2presents the torque difference (MHdML) of the silica-filled SMR-L compounds at various ALK and APTES loadings. The incorporation of 1.0 phr of these additives into silica-filled SMR-L compounds produced compounds with a higher torque difference, compared to the control compound. The addition of up to 5.0 phr of these additives increased the torque difference; however, it was decreased with further increase of these additives. It was also evident that the minimum torque (ML) decreased with increase of the loading of these additives. The minimum torque rep-resented thefiller-filler inter agglomeration[13], and the value was used to measure the relative viscosity of a rubber compound[24]. The lower the value, the weaker thefi
ller-filler interaction became; resulting in lower viscosity of the compound. The incorporation of these additives into
silica-filled SMR-L compounds reduced the filler-filler interac-tion, which led to a lower viscosity that enhanced the processability of the silica-filled SMR-L compounds.
Table 1
The compound designation and formulation of silica-filled SMR-L compounds with ALK and APTES.
Ingredientsa Designation and composition of the SMR-L compound-based recipes
Un-filled Control ALK1 ALK3 ALK5 ALK7 APTES1 APTES3 APTES5 APTES7
SMR-L 100.0 100.0 100.0 100.0 100.0 100.0 100.0 100.0 100.0 100.0
ZnO 5.0 5.0 5.0 5.0 5.0 5.0 5.0 5.0 5.0 5.0
Stearic acid 2.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0
IPPD 2.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0
MBTS 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5
Sulphur 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5
Vulcasil-S e 30.0 30.0 30.0 30.0 30.0 30.0 30.0 30.0 30.0
ALK e e 1.0 3.0 5.0 7.0 e e e e
APTES e e e e e e 1.0 3.0 5.0 7.0
aParts per hundred parts of rubber.
Fig. 2.Scorch times (ts2) and cure times (t90) of the silica-filled SMR-L
compounds at various ALK and APTES loadings.
In theory, the torque difference may be used as an indication of the crosslink density of a rubber compound [25e28]. The greater the torque difference value, the higher
the crosslink density. The total crosslink density was contributed by sulphide crosslinks and physical crosslinks [29e30]. The addition of up to 5.0 phr of these additives
into the silica-filled SMR-L compounds increased the tor-que difference of the SMR-L compounds. This was clearly attributed to the actions of these additives. As previously mentioned, the hydrophobic silica (due to the incorpora-tion of ALK and APTES) maintained the performance of zinc oxide in activating the MBTS, which accelerated the sulphur reaction and enhanced the state of the sulphide crosslink. The more hydrophobic silica became, the more compatible it was to SMR-L. As a consequence, the addition of these additives not only reduced thefiller-filler interac-tion, but also enhanced the rubber-filler interaction; lead-ing to the formation of coupllead-ing bonds between the additives with both silica and SMR-L. These coupling bonds were considered to be another type of crosslink, which contributed to the total crosslinks of silica-filled SMR-L compounds.
The reduction of the torque difference after the 5.0 phr of ALK and APTES loading was most probably attributed to the dilution effect of excessive amounts of additives, which lowered the crosslink density.
3.2. Effects of ALK and APTES on the silica dispersion
The degree of silica dispersion, with or without the addition of ALK and APTES in the SMR-L phase, was determined quantitatively by Equation(4) [17,31e32].
L¼hr mr (4)
where:
h
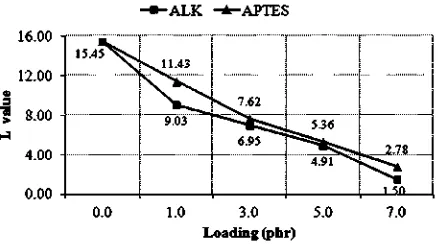
r¼[MLf/MLg], and mr¼[MHf/MHg]; where MLfand MHfwere the minimum and the maximum torques of the filled compounds; and MLgand MHgwere the minimum and the maximum torques of the unfilled/gum SMR-L compound. FromTable 2, MLg¼0.07 and MHg¼4.88. The lower the value of L at a particular silica loading, the better the silica dispersion became in the SMR-L phase.The value of L for the silica dispersion in the SMR-L phase is presented inTable 3andFig. 3. It is seen that the value of L of the silica dispersion in the rubber phase of the control compound was the highest. This was attributed to the surface of silica, which was saturated with hydrophilic silanol groups, and its relatively weak interaction with SMR-L. The silica particles also had a strong tendency to interact with each other and form large agglomerates[33]. Consequently, the dispersion of silica in the SMR-L com-pounds became poor. The addition of ALK and APTES at 1.0 phr into the silica-filled SMR-L compounds lowered the value of L. This was clearly due to the polar parts of these additive molecules that had a strong interaction with silica, which transformed the filler into a hydrophobic one. Consequently, silica became more compatible with SMR-L; hence improving the dispersion. The higher the loading of the additives, the lower the value of L; which meant enhanced silica dispersion.
The comparison of the L value of ALK with that of APTES is presented inTable 3andFig. 3. It is seen that at a similar loading, ALK caused a lower L value of silica in the SMR-L compound. This was attributed to the additional function of ALK as an internal plasticiser, which caused the reduc-tion of thefiller-filler interaction to lead to a betterfiller dispersion compared to APTES. This explanation was in line with the data inTable 2. At a similar loading, the minimum torque (ML) of ALK was lower than that of APTES. Table 2
Torque differences of silica-filled SMR-L compounds at various ALK and APTES loadings.
Cure characteristics Designation of the SMR-L compounds based recipes
Un-filled Control ALK1 ALK3 ALK5 ALK7 APTES1 APTES3 APTES5 APTES7
MH, dN.m 4.88 10.34 10.29 11.40 11.60 9.40 10.43 10.90 11.48 9.43
ML, dN.m 0.07 1.23 0.78 0.65 0.51 0.24 0.95 0.69 0.54 0.33
MHeML, dN.m 4.81 9.11 9.51 10.75 11.09 9.16 9.48 10.21 10.94 9.10
Table 3
The value of L for silica dispersion in SMR-L compounds.
Dispersion parameter Designation of the SMR-L compounds based recipes
Control ALK1 ALK3 ALK5 ALK7 APTES1 APTES3 APTES5 APTES7
hr 17.57 11.14 9.29 7.29 3.43 13.57 9.86 7.71 4.71
mr 2.12 2.11 2.34 2.38 1.93 2.14 2.33 2.35 1.93
L¼hremr 15.45 9.03 6.95 4.91 1.50 11.43 7.62 5.36 2.78
Fig. 3.The L values of silica with ALK and APTES.
3.3. Effects of ALK and APTES on rubberefiller interactions
The rubber-filler interaction depends on the degree of the filler dispersion in the rubber phase. Better filler dispersion results in stronger rubber-filler interactions. The rubber-filler interaction, with the addition of ALK and APTES to the silica-filled compound (based on the Lorenz and Park's equation), is presented inFig. 4.
It is shown that Qf/Qg decreased with increase of the ALK and APTES loading, up to 5.0 phr, and then increased with further increase to the loading. The decrease in Qf/Qg indicated that the SMR-Lesilica interactions became stronger with the addition of ALK and APTES. This was attributed to the ability of these additives to chemically modify the surface of silica, which was then more compatible with SMR-L; hence, improving the wetting/ dispersion of silica and, consequently, improving the SMR-Lesilica interaction. This explanation is in agreement with Fig. 3.
The increase of Qf/Qg after 5.0 phr loading was probably largely due to the excessive loading forming a layer in the silica-filled SMR-L system. The layer absorbed, coated and trapped the silica reducing the rubber-filler interaction.
At a similar loading, the Qf/Qg values of ALK were lower than those of APTES. Again, this was attributed to the additional function of ALK as an internal plasticiser, which caused betterfiller dispersion and led to a stronger
rubber-filler interaction.
3.4. Effects of ALK and APTES on reinforcing efficiency of silica
The degree of reinforcement provided by thefiller was calculated through its reinforcing efficiency (RE), which in its simplest form is given by Equation5 [31].
RE¼ ðMH MLf ðMH MLg=ðMH MLg (5)
in which:
(MHeML)f¼difference in torque value offilled compound (MHeML)g ¼ difference in torque value of unfilled/gum compound
High RE meant a high rubber-filler interaction, which was influenced by the degree offiller dispersion. Enhanced
filler dispersion provided a greater surface area for
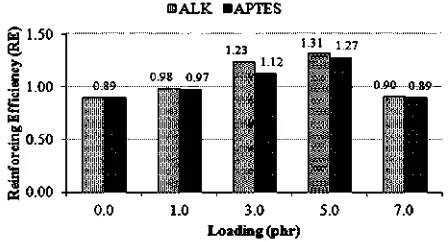
rubber-filler interactions. RE of silica on SMR-L, due to the addition of ALK and APTES into the silica-filled SMR-L compounds, is shown inFig. 5.
As presented in Fig. 5, ALK and APTES, with various loading, increased the RE of silica on the SMR-L. This is associated with the function of the additives as surface modifiers of silica that improved its compatibility with SMR-L; hence, improving the dispersion and rubber-filler interaction.
At a similar loading, ALK caused a higher RE of silica. This was due to better dispersion of silica and a stronger interaction of SMR-Lesilica in the presence of ALK as compared to APTES. This explanation is in agreement with the results inFigs. 3 and 4.
3.5. Effects of ALK and APTES on mechanical properties
The effects of ALK and APTES on the M100, M300, hardness, EB, resilience and TS of silica-filled SMR-L com-pounds are shown inFigs. 6, 7, 8, 9, 10 and 11, respectively. The incorporation of up to 5.0 phr of ALK and APTES into the silica-filled SMR-L compounds increased the tensile modulus (M100 and M300), but modulus decreased with further increase in the loadings. The results of hardness, tensile strength and resilience also exhibited a similar trend.
Since the tensile modulus of a rubber vulcanisate is only dependent on the degree of crosslinking [34e35], the
enhancement of tensile modulus to 5.0 phr was attributed to a higher crosslink density due to the formation of coupling bonds between the additives and both silica and SMR-L. The coupling bonds were another type of crosslink [2], which contributed to the total crosslink density of the SMR-L compounds. Therefore, with the addition of the additives, their effects were the same as an increase in crosslink density. This explanation is in line with the results inTable 2. The silica-filled SMR-L compounds with addi-tives had a higher value of (MH ML) than the silica-filled vulcanisate without additives (control compound).
The deterioration of the tensile modulus beyond 5.0 phr was attributed to the excessive loading of these additives, which caused a lower crosslink density. Presumably, the excessive amounts of these additives formed boundary layers which dissolved and coated part of the elemental curatives and silica particles, leading to decreased
Fig. 4.The Qf/Qg values of silica with ALK and APTES. Fig. 5.Reinforcing efficiency of silica with ALK and APTES.
formation of both sulphide and coupling bond crosslinks. Again, this explanation is in line with the data inTable 2.
As presented inFigs. 6 and 7, at a similar loading, ALK caused a decrease in M100 and an increase in M300, compared to the silica-filled SMR-L compounds with APTES. A decrease in M100 was attributed to the plasti-cising effect of ALK, which modified the modulus/stiffness property. A plasticiser may be used, not only to improve the rubber compound processing, but also to modify physical properties (such as stiffness and flexibility) of a rubber vulcanisate[36].
An increase in M300 was attributed to a stronger rubber-filler interaction due to the plasticising effect of
ALK. The ALK lowered the viscosity of the silica-filled SMR-L compound, which led to better filler dispersion and greater rubber-filler interaction. M300 displayed the de-gree of rubber-filler interactions[37e38]. This explanation
is in line with the data inFig. 4, which demonstrated that the Qf/Qg values of ALK were lower than those of APTES.
As presented in Fig. 8, hardness showed a similar behaviour as M100, which displayed the stiffness of a rubber vulcanisate[39]. Like tensile modulus, hardness also depends solely on the degree of crosslinks[34e35]. The
enhancement of hardness up to 5.0 phr was attributed to a higher crosslink density, and the deterioration of hardness beyond 5.0 phr was attributed to a lower crosslink density. At a similar loading, the hardness of ALK was lower than that of APTES. This was due to the plasticising effect of ALK, which softened the silica-filled SMR-L vulcanisates.
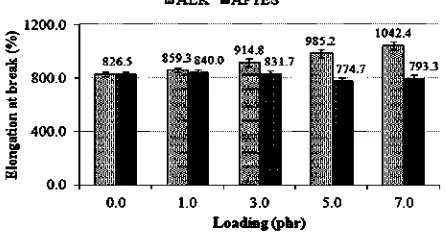
Fig. 9represented the effects of ALK and APTES on the elongation at break (EB) of the silica-filled SMR-L com-pounds. As seen, APTES reduced the EB of silica-filled SMR-L vulcanisate up to 5.0 phr of loading, and then increased slightly it as the loading further increased. EB depends mostly on the degree of crosslink density[35]. The reduc-tion of the EB up to 5.0 phr was simply attributed to a higher crosslink density, which immobilised the SMR-L segments from the silica surface. The increase of EB beyond 5.0 phr was attributed to a lower crosslink density. A contrary result was obtained when ALK was utilised. The EB increased with the increasing of ALK loadings. This was attributed to the function of ALK as an internal
Fig. 6.Modulus at 100% of the silica-filled SMR-L compounds at various ALK and APTES loadings.
Fig. 7.Modulus at 300% of the silica-filled SMR-L compounds at various ALK and APTES loadings.
Fig. 8.Hardness of the silica-filled SMR-L compounds at various ALK and APTES loadings.
Fig. 9.Elongation at break of the silica-filled SMR-L compounds at various ALK and APTES loadings.
Fig. 10.Resilience of the silica-filled SMR-L compounds at various ALK and APTES loadings.
plasticiser, which modified theflexibility of the silica-filled SMR-L compounds. As an internal plasticiser, ALK provided a free volume that allowed increased mobility/flexibility for the rubber chains to move.
Fig. 10displays the effects of ALK and APTES on the resilience of the silica-filled SMR-L compounds. It shows that APTES increased the resilience of silica-filled SMR-L compounds up to 5.0 phr of loading, and then decreased it as the loading further increased.
Like tensile modulus and hardness, resilience also de-pends on the number of crosslinks[36,39]. The enhance-ment of resilience up to 5.0 phr was attributed to a higher crosslink density, and the deterioration of resilience beyond 5.0 phr was attributed to a lower crosslink density. ALK presented a similar trend as APTES with loading up to 5.0 phr; however, beyond 5.0 phr, the ALK displayed a further increase in resilience. Again, this was attributed to the plasticising effect of the ALK, which improved the
flexibility of the silica-filled SMR compounds. According to Hofmann [39] and Ignatz-Hoover & To [36], rebound
resilience not only depends on the degree of crosslinks, but also on theflexibility of the rubber chains. The morefl ex-ible the rubber chains, the higher the resilience. The excessive amount of ALK (7.0 phr) caused a moreflexible silica-filled SMR chains.
At a similar loading, the resiliencies of ALK were higher than those of APTES. Again, this was attributed to the plasticising effect of ALK, which caused a higher crosslink density and a higher EB (moreflexibility).
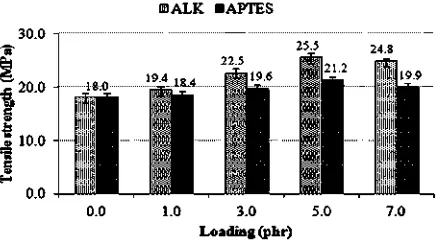
Fig. 11 illustrates the tensile strength of silica-filled SMR-L compounds at various ALK and APTES loadings. The tensile strength enhancement was attributed to the ability of ALK and APTES to improve the silicaeSMR-L in-teractions. The silica surface was saturated with hydro-philic silanol groups, which were relatively incompatible with SMR-L, and its interaction with SMR-L was relatively low. Both the silane coupling agent[40]and ALK[17]may chemically modify the surface of silica, and transform it into hydrophobic silica. This additive-modified silica was more compatible with SMR-L; as a result, the improved wetting/dispersion and the improved silicaeSMR-L inter-action led to the formation of physical crosslinks. These physical crosslinks further contributed to the total crosslink density[29e30]. The greater the physical crosslinks, the
higher the reinforcing efficiency of thefiller, and a higher tensile strength is produced.
At a similar loading, ALK produced a higher tensile strength than APTES. This may be attributed to a higher reinforcing efficiency of ALK due, for example, to better
filler dispersion and greater rubber-filler interaction. Ac-cording to Cohan[41], higher breaking elongation tends to give higher tensile strength. A higher EB meant a higher strain at break of the silica-filled SMR-L vulcanisate; which was delayed until a larger strain.
3.6. Scanning electron microscopy (SEM) study
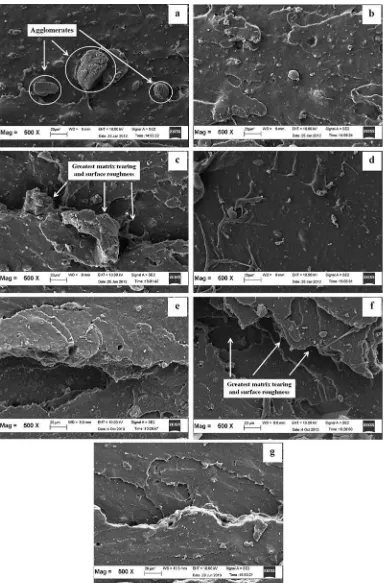
The SEM micrographs inFig. 12demonstrate the frac-tured surfaces of silica-filled SMR-L compounds with ALK and APTES at a magnification of 200X. The micrographs showed the improvement of the silica dispersion due to the addition of ALK and APTES. The dispersion of thefiller was the least homogeneous in (a), where large silica ag-glomerates were observed (indicated by arrows in Fig. 12a). The SEM micrograph of (a) also seemed relatively smooth compared to the others, which indicated that (a) was less ductile than the others. However, the SEM micrograph for (c) exhibited a comparable matrix tearing line and surface roughness with (f). Both (c) and (f) pre-sented the greatest matrix tearing line and surface roughness compared to the others (b, d, e and g). A greater rubber-filler interaction in both (c) and (f) altered the crack path, which led to increased resistance to crack propagation that caused an increase in tensile modulus, tensile strength and hardness. The micrographs of the tensile fractured surfaces were in mutual agreement with the results inFig. 4, which illustrated that the Qf/Qg values of ALK-5 and APTES-5 were the lowest ones. An enhancement in rupture energy, due to a greater
rubber-filler interaction, was responsible for the roughness and matrix tearing line of the fractured surface. The micro-graphs of the tensile fractured surfaces were in mutual agreement with the results obtained by other researchers [42e43]who reported that an increase in rupture energy
was responsible for the roughness and matrix tearing line of the fractured surface.
However, the matrix tearing lines and surfaces rough-ness of (d) and (g) were smoother than those of (c) and (f), which indicated lower crosslink densities.
4. Conclusions
From this study, the following conclusions were drawn:
1. Alkanolamide and aminopropyltriethoxy silane (APTES) acted as co-curing additives in silica-filled natural rub-ber compounds. Both of the additives increased the cure rate and torque difference.
2. A 5.0 phr loading of alkanolamide and amino-propyltriethoxy silane (APTES) was the optimum loading to improve the properties of silica-filled natural rubber compounds.
3. At a similar loading, alkanolamide indicated a higher degree of silica dispersion, greater silicaenatural rubber interaction and higher reinforcing efficiency than ami-nopropyltriethoxy silane (APTES).
Fig. 11.Tensile strength of silica-filled SMR-L compounds at various ALK and APTES loadings.
Fig. 12.SEM micrographs of the failed fracture of silica-filled vulcanisate at a magnification of 500x: (a) Control, (b) ALK3, (c) ALK5, (d) ALK7, (e) APTES3, (f) APTES5, and (g) APTES7.
Acknowledgements
The authors would like to thank Universiti Sains Malaysia for providing the research facilities for carrying out the experiment and for making this research work possible. One of the authors (Indra Surya) is grateful to the Directorate General of Higher Education (DIKTI), Ministry of Education and Culture (Kemdikbud) of the Republic of Indonesia, for the award of a scholarship under thefifth batch of the Overseas Postgraduate Scholarship Program.
References
[1]M.Q. Fetterman, The unique properties of precipitated silica in the design of high performance rubber, Elastomerics 116 (1984) 18e31.
[2]M. Fetterman, Precipitated silicaecoming of age, Rubber World 194
(1986) 38e40.
[3]P.E. Pinter, C.R. Mc. Gill, Comparing rubber fillers in an energy conscious economy, Rubber World International 177 (1978) 30e37.
[4]E.P. Plueddemann, in: E.P. Plueddemann (Ed.), Silane Coupling Agents, Plenum Press, New York, 1982, p. 4. Chapter 1.
[5]I. Gelling, M. Porter, A. Roberts, in: F.R. Eirich (Ed.), Natural Rubber Science and Technology, 1988, p. 367.
[6]F. Lautenschlaeger, K. Edwards, Model compound vulcanization-part V. The effect of chemical additives andfillers, Rubber Chemis-try and Technology 53 (1980) 27e47.
[7]M. Ranney, C. Pagano, Silane coupling agent effects in ethylene propylene diene terpolymers, Rubber Chemistry and Technology 44 (1971) 1080e1092.
[8]M. Wagner, Heat generation and rubber-filler coupling bonds, Rubber Chemistry and Technology 47 (1974) 697e716.
[9]A. Gent, E. Hsu, Coupling reactions of vinylsilanes with silica and poly (ethylene-co-propylene), Macromolecules 7 (1974) 933e936.
[10] U. Goerl, A. Hunsche, A. Mueller, H. Koban, Investigations into the silica/silane reaction system, Rubber Chemistry and Technology 70 (1997) 608e623.
[11] M. Fetterman, Filler effect on the heat stability of vulcanized elas-tomeric compositions, Rubber Chemistry and Technology 46 (1973) 927e937.
[12] M. Wagner, Reinforcing silicas and silicates, Rubber Chemistry and Technology 49 (1976) 703e774.
[13] A.K. Manna, P. De, D. Tripathy, S. De, D.G. Peiffer, Bonding between precipitated silica and epoxidized natural rubber in the presence of silane coupling agent, Journal of Applied Polymer Science 74 (1999) 389e398.
[14] P. Sae-Oui, C. Sirisinha, U. Thepsuwan, K. Hatthapanit, Comparison of reinforcing efficiency between Si-69 and Si-264 in a conventional vulcanization system, Polymer Testing 23 (2004) 871e879.
[15] P. Sae-Oui, C. Sirisinha, K. Hatthapanit, U. Thepsuwan, Comparison of reinforcing efficiency between Si-69 and Si-264 in an efficient vulcanization system, Polymer Testing 24 (2005) 439e446.
[16] P. Sae-oui, C. Sirisinha, U. Thepsuwan, K. Hatthapanit, Roles of silane coupling agents on properties of silica-filled polychloroprene, Eu-ropean Polymer Journal 42 (2006) 479e486.
[17] I. Surya, H. Ismail, A. Azura, Alkanolamide as an accelerator,fi ller-dispersant and a plasticizer in silica-filled natural rubber com-pounds, Polymer Testing 32 (2013) 1313e1321.
[18] O. Lorenz, C. Parks, The crosslinking efficiency of some vulcanizing agents in natural rubber, Journal of Polymer Science 50 (1961) 299e312.
[19] H. Ismail, M. Nasaruddin, U. Ishiaku, White rice husk ashfilled natural rubber compounds: the effect of multifunctional additive and silane coupling agents, Polymer Testing 18 (1999) 287e298.
[20] H. Ismail, S. Shaari, N. Othman, The effect of chitosan loading on the curing characteristics, mechanical and morphological properties of chitosan-filled natural rubber (NR), epoxidised natural rubber (ENR)
and styrene-butadiene rubber (SBR) compounds, Polymer Testing 30 (2011) 784e790.
[21] R. Mukhopadyay, S. De, Effect of vulcanization temperature and differentfillers on the properties of efficiently vulcanized natural rubber, Rubber Chemistry and Technology 52 (1979) 263e277.
[22] H. Long (Ed.), Basic Compounding and Processing of Rubber, Rubber Division, American Chemical Society Inc. The University of Akron, Ohio, USA, 1985.
[23] H.L. Stephens, The compounding and vulcanization of rubber, in: M. Morton (Ed.), Rubber Technology, Van Nostrand Reinhold, New York, 1987, pp. 20e58.
[24] H. Ismail, R. Nordin, A. Noor, Cure characteristics, tensile properties and swelling behaviour of recycled rubber powder-filled natural rubber compounds, Polymer Testing 21 (2002) 565e569.
[25] B. Boonstra, H. Cochrane, E. Dannenberg, Reinforcement of silicone rubber by particulate silica, Rubber Chemistry and Technology 48 (1975) 558e576.
[26] H. Cochrane, C. Lin, The influence of fumed silica properties on the processing, curing, and reinforcement properties of silicone rubber, Rubber Chemistry and Technology 66 (1993) 48e60.
[27] H. Ismail, C. Ng, Palm oil fatty acid additives (POFA's): preparation and application, Journal of Elastomers and Plastics 30 (1998) 308e327.
[28] P. Teh, Z. Mohd Ishak, A. Hashim, J. Karger-Kocsis, U. Ishiaku, Effects of epoxidized natural rubber as a compatibilizer in melt com-pounded natural rubbereorganoclay nanocomposites, European
Polymer Journal 40 (2004) 2513e2521.
[29] G. Kraus, Interactions of elastomers and reinforcingfillers, Rubber Chemistry and Technology 38 (1965) 1070e1114.
[30] K. Polmanteer, C. Lentz, Reinforcement studies-effect of silica structure on properties and crosslink density, Rubber Chemistry and Technology 48 (1975) 795e809.
[31] B. Lee, Reinforcement of uncured and cured rubber composites and its relationship to dispersive mixing-an interpretation of cure meter rheographs of carbon black loaded SBR and cis-polybutadiene compounds, Rubber Chemistry and Technology 52 (1979) 1019e1029.
[32] P. Pal, S. De, Effect of reinforcing silica on vulcanization, network structure, and technical properties of natural rubber, Rubber Chemistry and Technology 55 (1982) 1370e1388.
[33] N. Hewitt, Processing technology of silica reinforced SBR, Elasto-merics, (March, 1981) 16 (1981).
[34] D.L. Hertz Jr., S.E. Inc, theory&practice of vulcanization, Elasto-merics (November, 1984).
[35] H. Ismail, H. Chia, The effects of multifunctional additive and epoxidation in silica filled natural rubber compounds, Polymer Testing 17 (1998) 199e210.
[36] B. Rodgers, Rubber Compounding: Chemistry and Applications, CRC, 2004.
[37] G. Kraus, Reinforcement of elastomers by particulatefillers, Science and Technology of Rubber (1978) 387e416.
[38] S. Wolff, Optimization of silane-silica OTR compounds. Part 1: var-iations of mixing temperature and time during the modification of silica with bis-(3-triethoxisilylpropyl)-tetrasulfide, Rubber Chemis-try and Technology 55 (1982) 967e989.
[39] W. Hofmann, F. Bayer, Vulcanization and Vulcanizing Agents, Maclaren, 1967.
[40] M.A. Lutz, K.E. Polmanteer, H.L. Chapman, Novel wet-process silica prepared from alkyl silicates. Part I: synthesis, Rubber Chemistry and Technology 58 (1985) 939e952.
[41] L.H. Cohan, The mechanism of Re€enforcement of elastomers by pigments, Rubber Chemistry and Technology 21 (1948) 667e681.
[42] H. Ismail, M. Mathialagan, Comparative study on the effect of partial replacement of silica or calcium carbonate by bentonite on the properties of EPDM composites, Polymer Testing 31 (2012) 199e208.
[43] H. Nabil, H. Ismail, A. Azura, Compounding, mechanical and morphological properties of carbon-black-filled natural rubber/ recycled ethylene-propylene-diene-monomer (NR/R-EPDM) blends, Polymer Testing 32 (2013) 385e393.