Summary The effects of increased CO2 and temperature on the photosynthetic capacity of Siberian white birch and Japa-nese white birch (Betula platyphylla Sukatch. and B. platy-phylla Sukatch. var. japonica Hara) were measured. Birch seedlings were raised with a CO2 partial pressure of 36 ± 0.3 Pa (i.e., ambient) or 70 ± 0.6 Pa at day/night temperatures of either 30/16 °C or 26/12 °C. Siberian birch leaves were smaller and thicker than Japanese birch leaves. Water use efficiency and nitrogen use efficiency of Siberian birch grown in the CO2-enriched air were higher than those of Japanese birch. Both species showed a physiological adjustment to the growth CO2 partial pressure. Carboxylation efficiency and quantum yield of both species grown in CO2-enriched air were lower than those of seedlings grown in ambient CO2. The adaptation of Siberian and Japanese birch to elevated CO2 and temperature are discussed in relation to predicted climate change.
Keywords: carboxylation efficiency, climate change, eastern Siberia, foliar nitrogen concentration, photosynthetic adjust-ment, quantum yield, water use efficiency.
Introduction
White birch is a pioneer species that quickly becomes estab-lished following fire or other site disturbance (Koike and Sakagami 1985, Kuusela 1990, Ohta et al. 1993a). The species is broadly distributed in northeastern Asia (Tabata 1966, Gor-chakovsky and Shiyatov 1978), but precipitation and tempera-ture appear to be important factors affecting the distribution of white birch varieties. The Siberian variety grows in regions with low precipitation (200 mm per year), especially during the growing season (Ohta et al. 1993b, Ivanov 1994). The Japanese variety of white birch grows in regions with an annual precipitation of 1200--2000 mm (JFTA 1981).
Models of global climate change (Cubasch and Cess 1990) predict an increase in ambient temperature of 3 to 5 °C around mid and high latitudes (e.g., Woodward 1991, Oikawa 1993). Associated with this warming trend, the pattern and amount of precipitation in eastern Asia may change (Ohta et al. 1993b). Because Siberian birch is better adapted to dry sites than
Japanese birch, and the northern and southern limits of this broad ranging species are closely related to the cumulative temperature during the growing season, we postulated that a drier climate resulting from the predicted increases in atmos-pheric CO2 and temperature may increase the range of Siberian birch.
To test this hypothesis, we compared (1) the photosynthetic acclimation of Siberian and Japanese white birch to ambient and elevated CO2 during growth, and (2) the physiological and growth responses of these birch varieties to elevated CO2 and temperature.
Materials and methods
Plant materials
Seeds of Siberian birch (Betula platyphylla Sukatch.) and Japanese birch (B. platyphylla Sukatch. var. japonica Hara) were collected at Spasskaya Pad field station of the Yakut Institute of Biology (63° N, 129° E) and Hitsujigaoka experi-ment forest of the Forestry and Forest Products Research Institute (FFPRI, 43° N, 132° E), respectively. All seeds were germinated on vermiculite in ambient CO2 at day/night tem-peratures of 25/20 °C and a 22-h photoperiod. When seedlings were 8 cm in height, each was transplanted to a vinyl pot (diameter 15 cm, depth 12 cm) containing a 5/1 (v/v) mix of clay loam and pumice, and placed in the FFPRI phytotron in one of four treatment regimes.
Treatments
Ten seedlings were assigned to each CO2 × temperature treat-ment regime. The treattreat-ments were 36 ± 3 Pa CO2 (ambient) or 70 ± 5 Pa CO2 (elevated) at a day/night temperature of 30/16 (high temperature) or 26/12 °C (low temperature). The CO2 partial pressure was regulated with a CO2 controller (DAIWA Air Co. Ltd., Sapporo, Japan). The low temperature regime was based on the mean maximum and minimum temperatures from June to August at Spasskaya Pad and from June to September at Hitsujigaoka. The high temperature regime was set at 4 °C above these mean maximum and minimum
tem-Comparison of the photosynthetic capacity of Siberian and Japanese
birch seedlings grown in elevated CO
2
and temperature
T. KOIKE,
1,2T. T. LEI,
1T. C. MAXIMOV,
3R. TABUCHI,
1K. TAKAHASHI
1and B. I. IVANOV
31 Forestry and Forest Products Research Institute, Sapporo 062, Japan
2
Present address: Forest Environment Research, Tokyo University of Agriculture and Technology, Fuchu, Tokyo 183, Japan 3 Yakut Institute of Biology, The Russian Academy of Sciences, 677891 c. Yakutsk, Republic of Sakha
Received June 1, 1994
peratures. Day length was extended to 20 h with supplemental lighting (SOD metal-halide lamps, 500 W, Hitachi, Japan) to simulate the photoperiod of early spring. Liquid fertilizer (5/10/5 N,P,K, Hyponex, O.M. Scott & Sons Co., Marysville, USA) was supplied at the rate of 25 mg N l−1 week−1 pot−1. The trays containing the potted plants were filled with water to a depth of 1.5 cm each day.
Measurements
All measurements were made in an illuminated growth cabinet (Koito KG, Yokohama, Japan). The light source consisted of 16 metal-halide, six mercury, eight incandescent, and 16 fluo-rescent lamps. Light- and CO2-dependent photosynthetic rates at a leaf temperature of 27.2 °C were measured with a portable gas analyzer (ADC H3, Hoddesdon, U.K.). A supplemental halogen lamp (Nikon, Tokyo, Japan) was used to generate irradiances above 1200 µmol m−2 s−1. The photosynthetic photon flux density (PPFD) incident on the surface of the leaf chamber was regulated with neutral density cloth filters (Kurary Co., Osaka, Japan). The CO2 partial pressure was varied by mixing CO2-enriched air and soda-lime filtered air with a flow meter (Kojima Sci. Co., Tokyo, Japan). Gas ex-change rates were measured on the third and fourth leaves from the shoot tip. The age of measured leaves was always 25 to 30 days. Four or more replicates were used for each measurement. Quantum yield (φ) was estimated from the initial slope of the light--photosynthesis curve determined at CO2 saturation. Car-boxylation efficiency (CE) was calculated by the initial incre-ment of intercellular CO2 partial pressure (Ci) and net photosynthetic rate based on the assumption of uniform sto-matal responses to CO2 (Terashima 1992). Maximum photo-synthetic rate at saturating light and CO2 (Pmax ) was determined to estimate the rate of ribulose-1,5-bisphosphate regeneration (Farquhar and Sharkey 1982). Water use effi-ciency (WUE, µmol mmol−1) was calculated as the ratio of photosynthesis to transpiration at saturating light and constant water vapor pressure deficit (Field et al. 1983). Relative sto-matal limitation of photosynthesis (ls, %) was estimated from
ls = (Ao − A)/Ao, where Ao is the net photosynthetic rate when stomatal resistance to CO2 diffusion is zero, and A is the actual photosynthetic rate (Sharkey 1985). After the gas exchange measurement, leaf area was determined with an area meter (Hayashi AAM-5, Tokyo, Japan), and leaves were oven dried at 85 °C for 48 h. Specific leaf area (SLA) was calculated as leaf area per dry mass (cm2 g−1). Leaf nitrogen concentration was analyzed with a N/C analyzer (Yanagimoto MT 500W, Tokyo, Japan). Chlorophyll concentration of leaves was deter-mined after extraction with 80% ethanol (Arnon 1949).
Results
Foliage characteristics
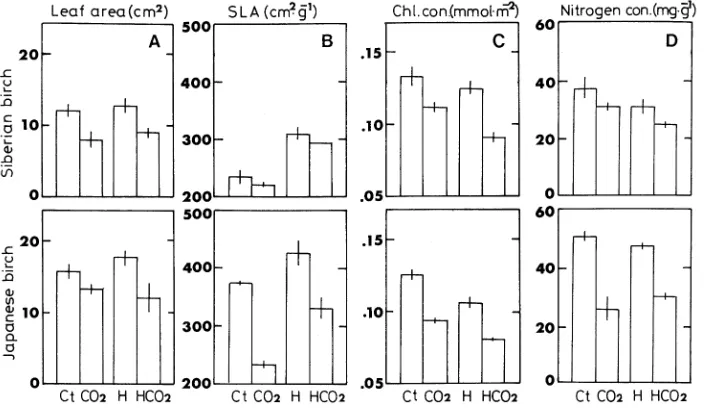
Cessation of leader shoot growth occurred about 45 and 65 days after leaf unfolding for Siberian birch and Japanese birch, respectively. The number of leaves per leader shoot was 10.4 ± 2.4 for Siberian birch and 17.6 ± 2.2 for Japanese birch. The mean leaf size of Siberian birch was 20% less than that of Japanese birch. The temperature treatment did not affect leaf area; however, the elevated CO2 treatment reduced mean leaf size of both birch varieties (Figure 1A). Siberian birch had a smaller SLA than Japanese birch in the 70 Pa CO2 plus low temperature treatment (Figure 1B). The elevated CO2 treat-ment decreased leaf chlorophyll concentration and leaf nitro-gen concentration of both birch varieties, and the effect on leaf nitrogen concentration was greater in Japanese birch than in Siberian birch (Figures 1C and 1D). The high temperature treatment decreased foliar chlorophyll concentration but had no effect on foliar nitrogen concentration in Siberian birch.
Photosynthetic characteristics in leaves
The elevated CO2 treatment decreased the quantum yield (φ) of both birch varieties (Figure 2A). The value of φ was strongly linked with leaf chlorophyll concentration (cf. Figures 1C and 2A). The elevated CO2 treatment suppressed Pmax of both birch varieties, but Siberian birch had a slightly higher Pmax than
Japanese birch (Figure 2B) in the low temperature plus 70 Pa CO2 treatment. In response to the elevated CO2 treatment, CE was marginally reduced in Siberian birch and markedly re-duced in Japanese birch (Figure 2C). Nitrogen-based photo-synthetic rates were higher in seedlings grown at 70 Pa CO2 than at 36 Pa CO2 in both birch varieties (Figure 2D), and higher in Siberian birch than in Japanese birch.
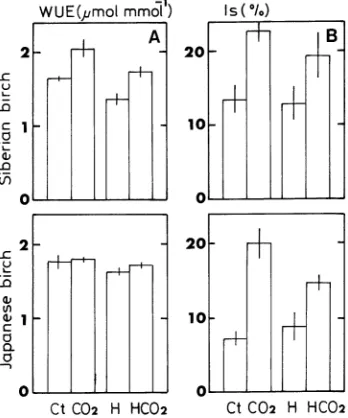
Independent of treatment temperature, the elevated CO2 treatment significantly enhanced WUE and stomatal limitation (ls) of Siberian birch, whereas in Japanese birch, the elevated CO2 treatment had no effect on WUE (Figure 3A) but signifi-cantly increased ls (Figure 3B).
Homeostatic adjustment in photosynthesis
At a given measurement CO2 concentration, net photosyn-thetic rate was always lower in seedlings grown in elevated CO2 than in seedlings grown in ambient CO2 irrespective of growth temperature. However, as shown by the dotted lines connecting the triangles in Figure 4, net photosynthetic rates did not differ between seedlings grown and measured at 36 Pa CO2 and seedlings grown and measured at 70 Pa CO2,
indicat-Figure 2. Photosynthetic charac-teristics of Siberian birch and Japa-nese birch plants grown at 36 or 70 Pa CO2 at a day/night temperature of 26/12 or 30/16 °C. Symbols: φ = quantum yield of light--photosyn-thesis curve; Pmax = maximum photosynthetic rate realized at satu-rated CO2 and PPFD; CE = car-boxylation efficiency; Pn = net photosynthetic rate per leaf nitro-gen concentration; Ct = 26/12 °C + 36 Pa; CO2 = 26/12 °C + 70 Pa; H = 30/16 °C + 36 Pa; and HCO2 = 36/16 °C + 70 Pa.
Figure 3. Water use efficiency (WUE) and stomatal limitation (ls) of Siberian birch and Japanese birch grown at 36 or 70 Pa CO2 at a day/night temperature of 26/12 or 30/16 °C. Symbols: Ct = 26/12 °C + 36 Pa; CO2 = 26/12 °C + 70 Pa; H = 30/16 °C + 36 Pa; and HCO2 = 36/16 °C + 70 Pa.
ing homeostatic adjustment. The high temperature growth regime resulted in increased maximum photosynthetic rates for both birch varieties, and the increase was greater for Sibe-rian birch than for Japanese birch.
Discussion
The patterns of morphological and physiological acclimation of Siberian birch and Japanese birch to elevated CO2 partial pressures were similar to those reported for other tree species (e.g., Eamus and Jarvis 1989, Bazzaz 1990). We observed decreases in chlorophyll concentration and foliage nitrogen concentration and an increase in nitrogen use efficiency in response to elevated CO2 (Figures 1 and 2). Sage et al. (1989) demonstrated that the photosynthetic rate of five plant species grown in elevated CO2 was regulated by leaf-level nitrogen. Tissue et al. (1993) concluded that the decline in the photosyn-thetic rate of loblolly pine seedlings grown for 2 years in a CO2-enriched atmosphere was caused by decreases in nitrogen and Rubisco content rather than by inactivation of Rubisco.
Both the Siberian birch and Japanese birch seedlings exhib-ited photosynthetic adjustment, i.e., net photosynthesis of seedlings grown and assayed at 70 Pa CO2 was similar to that of seedlings grown and assayed at 36 Pa CO2 (Figure 4). Photosynthetic adjustment to the growth CO2 partial pressure has also been reported in Alaskan perennials (Oechel and Strain 1985, Tissue and Oechel 1987) and French bean (Socias et al. 1993). Based on reciprocal transplanting experiments, it has been concluded that net photosynthetic rate adjusts to the growth CO2 concentration within 3 weeks (Tissue and Oechel 1987, Grulke et al. 1990, Oechel and Billings 1992).
Because photosynthetic rate is strongly influenced by the balance of source--sink activities (Thomas and Strain 1991), it has been suggested that photosynthetic adjustment is an arti-fact caused by pot size (Arp 1991). In a CO2-enriched atmos-phere, the depression in photosynthetic capacity is closely related to a deficiency of inorganic phosphate, which may be associated with the translocation of photosynthates (Sage et al. 1989, Sawada and Usuda 1992, Makino 1994). However, ho-meostatic adjustment of arctic tussock sedge occurred even though there was no root restriction (Oechel and Billings 1992). McConnoughay et al. (1993a, 1993b) concluded that growth of annual plants raised in a CO2-enriched atmosphere was controlled not by pot size but by the availability of nutri-ents.
Tissue et al. (1993) reported that an increased supply of nitrogen fertilizer enabled a high photosynthetic rate to be maintained in loblolly pine seedlings grown at elevated CO2. Similar results have been reported for Betula pendula Roth. (Pettersson et al. 1993). Coleman et al. (1993) suggested that the CO2-induced reduction in plant nitrogen concentration was the result of dilution caused by accelerated plant growth. Because the reductions in foliar nutrient concentrations, espe-cially nitrogen and phosphorus, closely paralleled the reduc-tions in CO2 exchange rates in plants grown in elevated CO2, we postulate that, in white birch, reductions in foliar nitrogen and phosphorus underlie the homeostatic adjustments.
Siberian birch leaves were smaller and thicker than Japanese birch leaves and are, therefore, better adapted to the dry cli-mate in eastern Siberia (Bewley and Krochko 1982). These morphological characteristics were more pronounced in Sibe-rian birch seedlings grown at 70 Pa CO2 and high temperature than in seedlings grown at 36 Pa CO2 and low temperature. The high photosynthetic rate of Siberian birch seedlings may also confer an advantage by enabling this variety to compensate for the short growing season (< 3 months) in eastern Siberia (Ohta et al. 1993a, Ivanov 1994). The photosynthetic capacity of Siberian birch seedlings acclimated to elevated CO2, but not to high temperature. Seedlings of this birch variety did not accli-mate to the high temperature regime because their phenotype includes small leaves adapted to a dry environment and so the seedlings can maintain high WUE at high temperatures. In contrast, Japanese birch seedlings showed a relatively modest degree of acclimation to elevated temperature and CO2 in foliage morphology and photosynthesis. We conclude that, under infertile conditions, as a result of homeostatic adjust-ment in photosynthesis, growth responses of both Siberian birch and Japanese birch to elevated CO2 will be small.
Acknowledgments
We thank N. Solomonov and M. Fukuda for their kind arrangements under the Japan-Russia Joint Research program sponsored by the Japan Environmental Agency (G. Inoue). Thanks are also due to B.R. Strain and W.C. Oechel for providing references and invaluable com-ments. This report is a part of the special project on global change organized by the University of Tsukuba.
References
Arp, W.J. 1991. Effects of source--sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ. 14:869--875. Arnon, D.I. 1949. Copper enzymes in isolated chloroplasts.
Polyphe-nol oxidase in Beta vulgaris. Plant Physiol. 24:1--15.
Bazzaz, F.A. 1990. The response of natural ecosystems to the rising global CO2 levels. Annu. Rev. Ecol. Syst. 21:167--196.
Bewley, J.D. and J.E. Krochko. 1982. Desiccation-tolerance. Encyclo-pedia of Plant Physiology, Vol. 12B. Springer-Verlag, Berlin, pp 325--378.
Coleman, J.S., K.D.M. McConaughay and F.A. Bazzaz. 1993. Ele-vated CO2 and plant nitrogen-use: is reduced tissue nitrogen con-centration size-dependent? Oecologia 93:195--200.
Cubasch, U. and R.D. Cess. 1990. Processes and modelling. In Cli-mate Change: The IPCC Scientific Assessment. Ed. J.T. Houghton. Cambridge University Press, Cambridge, U.K., pp 69--92. Eamus, D. and P.G. Jarvis. 1989. The direct effects of increase in the
global atmospheric CO2 concentration on natural and commercial temperate trees and forests. Adv. Ecol. Res. 19:1--55.
Field, C., J. Merino and H.A. Mooney. 1983. Compromises between water-use efficiency and nitrogen-use efficiency in five species of California evergreens. Oecologia 60:384--389.
Farquhar, G.D. and T.D. Sharkey. 1982. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 33:317--345.
Gorchakovsky, P.L. and S.G. Shiyatov. 1978. The upper forest limit in the mountains of the boreal zone of the USSR. Arct. Alp. Res. 10:349--363.
Ivanov, B.I. 1994. Sakha-Japan joint studies: ecophysiological studies of woody plants of Yakutia. YIB, Botanic Garden, Yakutsk, Sakha Rep., Russia, 104 p.
JFTA. 1981. Forestry and forest industries in Japan. Wood-Products Stockpile Corp., Tokyo, 26 p.
Koike, T. and Y. Sakagami. 1985. Comparison of the photosynthetic responses to temperature and light of Betula maximowicziana and Betula platyphylla var. japonica. Can. J. For. Res. 15:631--635. Kuusela, K. 1990. The dynamics of boreal coniferous forests. SITRA,
Helsinki, 172 p.
McConnaughay, K.D.M., G.M. Berntson and F.A. Bazzaz. 1993a. Plant responses to carbon dioxide. Nature 361:24.
McConnaughay, K.D.M., G.M. Berntson and F.A. Bazzaz. 1993b. Limitations to CO2-induced growth enhancement in pot studies. Oecologia 94:550--557.
Makino, A. 1994. Biochemistry of C3-photosynthesis in high CO2. J. Plant Res. 107:79--84.
Oechel, W.C. and W.D. Billings. 1992. Effects of global change on the carbon balance of arctic plants and ecosystems. In Arctic Ecosys-tems in a Changing Climate. Eds. F.S. Chapin, III, R.L. Jefferies, J.F. Reynolds, G.R. Shaver, J. Svoboda and E.W. Chu. Academic Press, New York, pp 139--168.
Oechel, W.C. and B.R. Strain. 1985. Native species responses to increased atmospheric carbon dioxide concentration. DOE/ER0238, USDE, pp 117−154.
Ohta, S., T. Koike, A. Osawa, T. Sassa and K. Takahashi. 1993a. On the survey of Taiga in the Siberian permafrost. J. For. Sci. 7:68--73 (in Japanese).
Ohta, Sh., Z. Uchijima and Y. Oshima. 1993b. Probable effects of CO2-induced climatic changes on net primary productivity of ter-restrial vegetation in East Asia. Ecol. Res. 8:199--213.
Oikawa, T. 1993. Atmospheric CO2: trends in biosphere. Climate Res. Note 181:91--109 (in Japanese).
Pettersson, R., A.J.S. McDonald and I. Stadenberg. 1993. Response of small birch plants (Betula pendula Roth.) to elevated CO2 and nitrogen supply. Plant Cell Environ. 16:1115--1121.
Sage, R.F., T.D. Sharkey and J.R. Seemann. 1989. Acclimation of photosynthesis to elevated CO2 in five C3 species. Plant Physiol. 89:590--596.
Sawada, S. and H. Usuda. 1992. The role of inorganic phosphate in activation of RuBPcase activity in response to changes in source/sink balance. In Research in Photosynthesis IV. Ed. N. Murata. Kluwer Academic Publishers, The Netherlands, pp 769--772.
Sharkey, T.D. 1985. Photosynthesis in intact leaves of C3 plants: physics, physiology and rate limitations. Bot. Rev. 51:53--105. Socisa, F.X., H. Medrano and T.D. Sharkey. 1993. Feedback limitation
of photosynthesis of Phaseolus vulgaris L. grown in elevated CO2. Plant Cell Environ. 16:81--86.
Tabata, H. 1966. A contribution to the biology of Japanese birches. Memorial Coll. Sci., Kyoto Univ., Ser. B. 32:239--271.
Terashima, I. 1992. Anatomy of non-uniform leaf photosynthesis. Photosynth. Res. 31:195--212.
Thomas, R.B. and B.R. Strain. 1991. Root restriction as a factor in photosynthetic acclimation of cotton seedlings grown in elevated carbon dioxide. Plant Physiol. 96:627--634.
Tissue, D.T. and W.C. Oechel. 1987. Response of Eriophorum vagi-natum to elevated CO2 and temperature in the Alaskan arctic tundra. Ecology 68:401--410.
Tissue, D.T., R.B. Thomas and B.R. Strain. 1993. Long-term effects of elevated CO2 and nutrients on photosynthesis and Rubisco in loblolly pine seedlings. Plant Cell Environ. 16:859--865.