In the aqueous phase, there are many factors (such as pH, temperature, and ferrate concentration) on which ferrate stability depends. External factors such as light are not known to significantly affect the stability of ferrate (0.01 M) [9].
Inorganic removal by ferrates
Although they observed a decrease in the rate of cyanide oxidation in the presence of Zn(II), complete removal was achieved by Fe(VI). They observed that rapid oxidation of CN was observed in the CN-Cu and CN-Ni systems, while a relatively slower degradation of CN was observed in the mixed CN-Cu-Ni.
Organic matter and wastewater
Among the various metal complexes, zinc cyanide (CN) found in wastewater from metal processing industries had a significant impact on the environment. The presence of an organic ligand EDTA increased the removal of cyanide in Ni(II)-CN-EDTA, while only 60% removal was observed with Ni(II)-CN metal cyanide complex.
Ferrate as a disinfectant
- Effect of ferrate ions on bacteria
- Effect of ferrate ions on viruses
- Effect of ferrate ions on algae
- Effect of ferrate ions on microcystins
Faster kinetics for virus inactivation may be due to denaturation of the genome or transformation of capsid proteins [65]. Eutrophication of lakes and rivers has increased rapidly due to the discharge of nutrients into water bodies, especially phosphates.
Conclusions
Development of ferrate(VI) salts as oxidant and coagulant for water and wastewater treatment. Advances in the development and use of ferrate (VI) salts as an oxidant and coagulant for water and wastewater treatment.
Introduction
In addition to breaking down extra chemical compounds directly, AOPs are used to break down cellular content to disinfect wastewater with a high organic nature in the dairy industry [8]. Complementing previous reviews [1, 9], this review categorizes relevant AOP applications in the food sector in winery, olive oil mill, meat industries, dairy and food coloring departments.
Introduction to current wastewater treatment technologies in the food industry
- Evaporation
- Land application
- Physicochemical treatments
- Membrane technologies
- Summary
Current technological alternatives to AOPs for wastewater treatment in the food industry are briefly introduced. Advances in wastewater treatment in the food industry are driven by the growing public awareness of environmental protection.
Application of AOPs in the food industry 1 Wineries
- Olive oil mills
- Meat processing industry
- Miscellaneous food industry wastewaters
- Food dyes
For the photo-Fenton process operating conditions in terms of pH, H2O2 concentration and catalyst dosage were optimized, yielding 90% of TOC removal with 54% due to adsorption and 36% due to the photo-Fenton process. Rich in nutrients and color, more OMW is degraded by the Fenton process in combination with pretreatment technologies after 2016 compared to before. For further investigation, various combinations of conventional pretreatment methods and Fenton process can be analyzed.
RSM has been applied to optimize the operating condition of the electro-Fenton process with iron electrodes [47]. Diluted effluent from real citrus juice was investigated with different ozonation processes, and it was found that the solar Fenton process is superior both in terms of removal efficiency and economic perspective [56]. The Fenton process was found to provide rapid OH production and stability despite the interference of real water matrices [61].
Practical application of AOPs
Azo compounds are widely used in the food industry due to their brilliant hues, relatively low cost, and ease of fabrication [60]. However, azo compounds are resistant to conventional processing due to one or more azo linkages [61]. To avoid the pH limitation of Fenton processes, catalysts have been synthesized with immobilized iron ions to prevent precipitation.
Conclusion
High-speed aerobic treatment of basement wastewater using free and immobilized activated sludge bioreactors. Integrated electrocoagulation-electrooxidation process for the treatment of soluble coffee effluent: Optimization of COD degradation and operation time analysis. Treatment of a mixture of food color additives (E122, E124 and E129) in different water matrices by UVA and solar energy. degradation of the artificial food azo-dye Ponceau 4R by advanced.
Treatment of winery wastewater by ozone-based advanced oxidation processes (O3, O3/UV and O3/UV/. H2O2) in a pilot-scale bubble column reactor and process economics. In the last part of the chapter, the development trends of catalytic ozone oxidation of petrochemical secondary wastewater will also be discussed. Therefore, the catalytic ozone oxidation process is widely used in the advanced treatment of petrochemical wastewater.
Research progress of catalytic ozone oxidation
Homogeneous catalytic oxidation
Advanced oxidation processes (AOPs) can produce hydroxyl radicals (∙OH) with strong oxidizing ability under the reaction conditions of electricity, sound, photo, catalyst and others. The ozone catalytic oxidation process is a kind of advanced oxidation process, which has the following characteristics: (a) it produces strong oxidizing ∙OH, and the oxidation process is fast, reaching 106 to 1010L/(mol∙s); (b) it performs excellently in the removal of refractory organic matter without secondary pollutants, meaning the process is green and efficient; and (c) the process is economical. 2. A complex coordination reaction takes place between the catalyst and the organic matter or ozone to promote the reaction between ozone and organic matter [2, 8, 9].
The free radical mechanism is similar to the Fenton reaction, and the electron transfer is achieved by oxidation and reduction of the valence metal ions. Furthermore, it was found that the reaction rate did not change in the presence of t-butanol (a radical scavenger). These suggest that the free radical mechanism is not responsible for mineralization of oxalate in the Co(II)/O3 system.
Heterogeneous catalytic oxidation
58], adsorption of organic molecule on the surface of MnO2 and subsequent attack of ozone on adsorbed organic molecule are responsible for catalytic activity. These could be the proof of the hypothesis that the catalytic activity of Al2O3 depends on the surface absorption of the substrate. The research showed that it was the high adsorption of oxalic acid on the surface that caused the lower efficiency.
As illustrated in Figure 3, the ozone molecule first forms a surface ring with the hydroxyl group on the surface of the catalyst and then decomposes to produce Fe-OH-O and O2. 2. Organic compounds form complexes on the surface of FeOOH and then directly oxidized by ozone molecules to break down organic matter [72]. Then, through the adsorption and desorption of organic matter on the surface of the catalyst, the electron transfer is completed to achieve metal reduction and.
Research on catalytic ozone oxidation in petrochemical secondary effluent
Research ideas
Results and discussion .1 The characteristic of catalyst
The highest removal efficiency (42.8%) of TOC was obtained in the ozone/catalyst system, followed by ozone/H2O2 (23.8%) and single ozone (4.7%). In the single ozone system, HoA showed the highest TOC removal, decreasing by 18.1%, whereas HiN showed the lowest content of TOC removal, which conversely increased by 12.0% in the effluent. As shown in Figure 10, the Φi/TOC ratio of HiN did not change significantly in the single ozone system, but gradually increased in the ozone/H2O2 and ozone/catalyst systems.
This also indicated that HiN was mainly responsible for DOM removal in the ozone/H2O2 system and especially in the ozone/catalyst system. Because HiN is the dominant fraction in the sample and was resistant to ozone, for the best treatment of petrochemical wastewater, efficient removal of HiN is necessary. The adsorption of organic matter on the catalyst surface and the simultaneous generation of ∙OH at the solid–liquid interface can guarantee the high consumption of ∙OH reacting with HI, preventing the emission of ∙OH into the bulk solution.
Problems on catalytic ozone oxidation in the PSE .1 The effect of flocs on catalytic ozone oxidation
93] indicate that esters, alcohols, olefins, ketones and nitrogen-containing organic compounds in PSE can be effectively removed by catalytic ozone oxidation. Twelve main characteristic organic pollutants are detected in the influent, about six kinds of which can be degraded by catalytic ozone oxidation. But the target characteristic pollutants in the wastewater can be reduced to three if the PSE is filtered by 0.45μm membrane before catalytic ozone oxidation.
Studies have shown that colloidal organic matter accounted for about 9%–15% of COD in PSE [94]. This is because the presence of colloidal macromolecular bodies in PSE affects the decomposition rate of ozone in the initial phase (t < 30 s), thereby reducing the efficiency of hydroxyl radical (∙OH) production [95]. Many studies have also shown that in advanced wastewater treatment, colloidal macromolecular organic matter mainly affected the removal of characteristic organic matter during ozone catalytic oxidation.
Conclusions
Study of catalytic ozone oxidation of humic substances in water and their ozonation by-products. Catalytic ozone oxidation for the degradation of 2,4,6-trichloroanisole in drinking water in the presence of gamma-AlOOH. Activated carbon and cerium oxide catalysts used for catalytic ozone oxidation of dyes and textile effluents.
FeOOH ozone catalytic oxidation of oxalic acid and the effect of phosphate binding on its catalytic activity. CuO/SBA-15 catalyst for ozone catalytic oxidation of mesoxalic and oxalic acids: Water matrix effects. Effect of wastewater particulates on catalytic ozone oxidation in advanced petrochemical secondary effluent treatment.
Scale-up of heterogeneous photocatalytic reactors
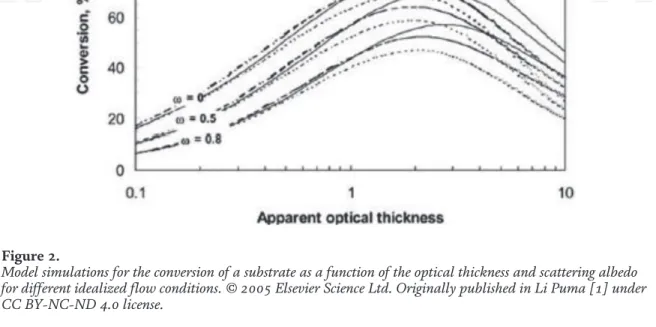
The optical thickness of the photoreactor (τ) corresponding to the degree of opacity of the photoreactor. The scattering albedo of the photocatalyst (ω), which depends on the optical properties of the catalyst. Both help to describe the dynamics of UV-photon absorption and determine the degree of difficulty of the upscaling process.
The dimensionless radiation intensity at the reactor entrance window and the dimensionless LVRPA may also be useful. Model simulations for the conversion of a substrate as a function of optical thickness and scattering albedo for different idealized flow conditions.©2005 Elsevier Science Ltd. conditions [3].
Optimization methods
However, this term cannot be applied to solar photoreactors due to the variability of solar incident radiation. This leads to the use of photoreactors with the same dimensions and hydrodynamic properties as those used for the experimental tests. Therefore, it is more advisable to evaluate the property to be optimized over a wide range of dependent variables.
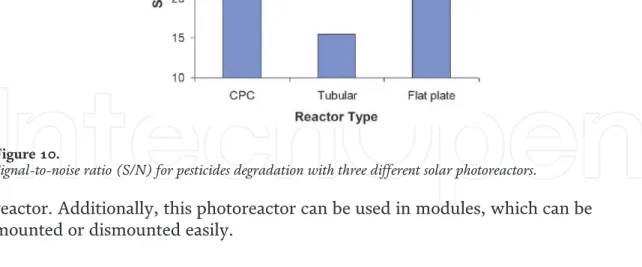
The graphs shown in Figure 6 describe the behavior of VRPA/H with tubular and CPC reactors with an internal diameter of 32 mm and AEROXIDE® P-25 as catalyst. It is important to note that these plots are only applicable for radiation conditions and the specified pipe diameter [7]. The presence of optima in each case is due to the increase in scattering with the optical thickness, which is usually associated with high catalyst loads that do not allow sufficient penetration of light into the interior of the reactor.

Design criteria
- Preliminary considerations
- Photoreactor selection
- Pilot-scale plant considerations
- Design, assembly, and operation of a full-scale solar photocatalytic plant This section presents the main aspects that were considered in the construction
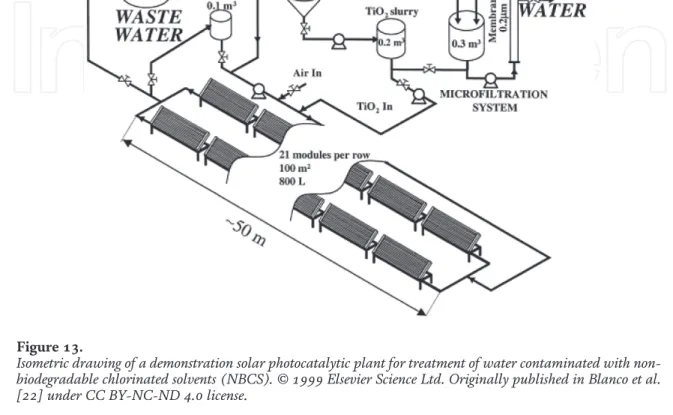
Furthermore, the type and concentration of the substrate are determinants for the performance of the photocatalytic reactor. The type, geometry and materials of construction of the photocatalytic reactor should remain the same for a full-scale plant. The process flow diagram of the full-scale solar photocatalytic plant is shown in Figure 14 .
The color was completely removed, confirming the satisfactory performance of the solar photocatalytic reactor. Based on these results, it was necessary to estimate the size of the full-scale plant for the treatment of 2 m3/d of wastewater. The apparent rate constants appear to be dependent on the accumulated energy and the type of day.
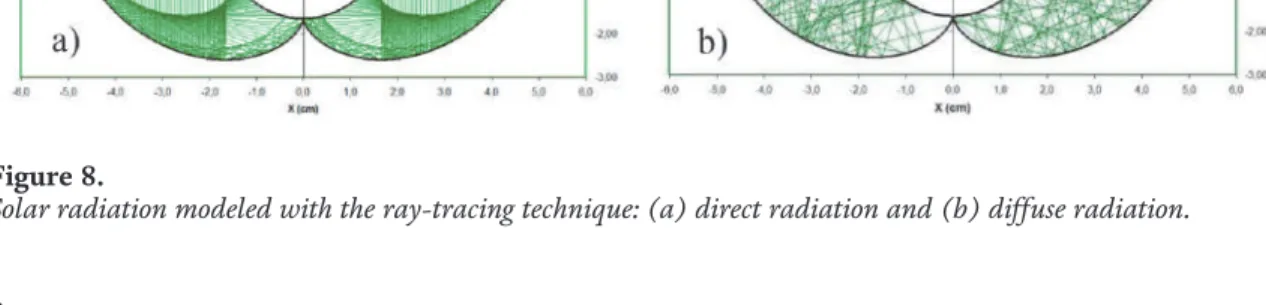
![Figure 3 shows an example where the surface response methodology was used for optimizing the decolorization of methylene blue (MB) with two bench-scale photoreactors [6].](https://thumb-ap.123doks.com/thumbv2/1libvncom/9202501.0/71.918.135.782.846.1250/figure-example-response-methodology-optimizing-decolorization-methylene-photoreactors.webp)