www.elsevier.com / locate / bres
Research report
Growing and regenerating axons in the visual system of teleosts are
recognized with the antibody RT97
a b a a
´
Almudena Velasco , Marıa Julia Bragado , David Jimeno , Elena Caminos ,
a a a ,
*
´
´
´
Concepcion Lillo , Jose Aijon , Juan M. Lara
a
´ ´ ´
Instituto de Neurociencias de Castilla y Leon, Departamento de Biologıa Celular y Patologıa, Universidad de Salamanca, E-37007 Salamanca,
Spain
b
´ ´ ´
Departamento de Fisiologıa, Universidad de Alcala de Henares, Madrid, Spain Accepted 2 August 2000
Abstract
We have analyzed the immunolabeling with the antibody RT97, a good marker for ganglion cell axons in several species, in the normal and regenerating visual pathways of teleosts. We have demonstrated that RT97 antibody recognizes several proteins in the tench visual system tissues (105, 115, 160, 200, 325 and 335 kDa approximately). By using immunoprecipitation and Western blot we have found that after crushing the optic nerve the immunoreactivity to anti RT97 increased markedly in the optic nerve. In immunohistochemical analysis we also found a different pattern of labeling in normal and regenerating visual pathways. In normal tench RT97 is a good marker for the horizontal cells in the retina, for growing ganglion cell axons which run along the optic nerve from the retina to the optic tectum and of the axon terminals in the stratum opticum and stratum fibrosum and griseum superficiale in the optic tectum. After optic nerve crush, no immunohistochemistry modifications were observed in the retina. However, in accordance with Western blot experiments, in the optic nerve intensely stained groups of regenerating axons appeared progressively throughout the optic nerve as far as the optic tectum. We conclude that the antibody RT97 is an excellent marker of growing and regenerating axons of the optic nerve of fish. 2000 Elsevier Science B.V. All rights reserved.
Theme: Development and regeneration
Topic: Regeneration
Keywords: Fish; Neurofilaments; Optic nerve; Optic tectum; Retina
1. Introduction levels, in the axonal transport and in the cytoskeleton of the ganglion cell axons [13,17].
The retinas of some teleosts have two peculiarities with In relation to the cytoskeleton of fish retinal ganglion respect to other vertebrates: the retina grows throughout cells, different studies of the expression of the proteins of the life of the animal from a peripheral germinal zone [26] the neurofilaments, both in the normal state and after a continuously adding new neurons and axons of the gang- lesion [9,14,22,29], have been carried out. In mammals, lion cells and the retinal ganglion cells are capable of principal neurofilament proteins have been classified into regenerating their axons and restoring vision after a lesion three types according to their molecular weight: neuro-in the optic nerve [4,17,31]. Durneuro-ing the growth and filament-light (NF-L), neurofilament-medium (NF-M) and regeneration processes important changes occur in the neurofilament-heavy (NF-H) [32]. In fish, differences in visual pathways, both at morphological and biochemical the composition of these proteins have been described in comparison to those of mammals. The predominant pro-teins of the neurofilaments of the goldfish optic nerve have a low molecular weight, such as gefiltin, a type IV
*Corresponding author. Tel.: 134-923-294-500; fax: 1
34-923-294-neurofilament protein [15] and plasticin, a type III
neuro-549.
E-mail address: [email protected] (J.M. Lara). filament protein [14]. NF-M proteins have also been
detected in the goldfish optic nerve with molecular weights with 3% H O in 100% methanol for 10 min. After rinsing2 2
of 145 kDa [16,30]. However, NF-H proteins have not twice for 10 min in phosphate buffer saline solution with been identified in the optic nerve of goldfish [13]. These 0.2% Triton X-100 (PBS–Tx), the sections were incubated differences with regard to the mammalian proteins appear overnight with RT97 monoclonal antibody (Hybridoma to be important in the continued plasticity of the visual Bank Iowa University) at a dilution of 1:1000 at 188C in a system [13]. Several of these proteins, such as plasticin humidity chamber, and subsequently incubated at room and gefiltin, modify their expression during regeneration temperature with biotinylated mouse IgG (Vector) at a [12,14,15,27,28,33]. Nevertheless, in the visual system of dilution of 1:200 and avidin–peroxidase complex (Vector) mammals [5,10,36] and several anamniotes, such as (1:250). The sections were finally reacted with 0.025% Xenopus and trout [6,38], proteins of NF-H in the axon 3.39-diaminobenzidine tetrahydrochloride (DAB) and ganglion cells have been detected. These proteins are also 0.025% hydrogen peroxide in 0.2 M Tris–HCl (pH 7.6) found in other areas of fish brains [1,9,22]. for 10 min at room temperature. Sections were dehydrated The antibody RT97 has been used to recognize the and mounted in Entellan (Merck). The right retinas and phosphorylated 200-kDa neurofilament sub-unit in mam- optic nerves of lesioned tench and the visual pathways mals [37]. Furthermore it also recognizes, though with (retinas, optic nerves and optic tecta) of unlesioned tench lesser affinity, another phosphorylated neurofilament pro- were used as controls. Immunohistochemical controls were tein of 155 kDa and also tau of Alzheimer neurofibrillary performed either by the omission of the primary antibody tangles [2,3,8,21] and the upper band of the MAP1B or by replacement with a non-immune serum.
doublet [19]. This antibody has also been used to label
specific populations of neurons in the rat [20,23]. In this 2.1. Immunoprecipitation /Western blot study, we analyze the distribution and modification of the
immunostaining of RT97 and which proteins are
recog-2.1.1. Tissue preparation nized by this antibody in the normal and damaged tench
Retina, optic nerve and optic tectum from control and visual system.
lesioned tench 30 days post-crushing were dissected and isolated. Tissue was sonicated twice at 48C for 5 s in lysis buffer which contained 50 mM Tris / HCl, pH 7.4, 150 mM
2. Material and methods
NaCl, 1% Triton X-100, 1 mM EGTA, 0.4 mM EDTA, 2.5
mg / ml aprotinin, 2.5 mg / ml leupeptin, 1 mM PMSF and We used 60 tench (Tinca tinca L., Teleostei), of body
0.2 mM Na VO . Tissue homogenates were centrifuged at3 4
length 15–20 cm, which were obtained from a commercial
15 000 g at 48C for 20 min. The soluble protein con-hatchery. The animals were anesthetized with 0.03%
centration was determined using BioRad Reagent. tricaine methanesulphonate (MS222, Sigma). The left optic
nerve was crushed 1 mm from the eyeball with a fine
2.1.2. Immunoprecipitation watchmaker’s forceps for about 3 s. The animals were kept
A total of 350 mg of protein in each sample of tissue in aquaria at 18–208C for periods from 1 to 200 days. At
were incubated over-night at 48C with 12ml of anti-RT97 least five animals were used at each different time point (2,
antibody or 12 ml of anti-MAP1B monoclonal antibody 7, 15, 30, 45, 60, 90, 120, 150 and 200 days) after crushing
(Clone AA6, RBI, USA). Immunocomplexes were precipi-the nerve. Animal manipulations were performed in
ac-tated with 25 ml of protein A conjugated to agarose cordance with the directives of the European Communities
(Upstate Biotechnology Inc., USA). When anti-MAP1B Council (86 / 609 / EEC) and current Spanish legislation
was used to immunoprecipitate, the same amount of a (BOE 67 / 8509-12, 1988) for animal care and
ex-rabbit anti-mouse IgG (Pierce) was added to increase the perimentation.
affinity of the immunocomplex for protein A-agarose. The The control and lesioned animals were re-anesthetized
immunoprecipitates were washed three times at 48C with and perfused transcardially with 0.68% NaCl solution
the lysis buffer containing proteases and phosphatase followed by 4% paraformaldehyde in phosphate buffer 0.1
inhibitors. Laemmli buffer was added to the immuno-M, pH 7.4 (PB). The retina, optic nerve and optic tectum
precipitates and the mixture was denatured by boiling. were dissected out and post-fixed for 4 h at 48C in the
same fixative solution. The sampled tissues were then
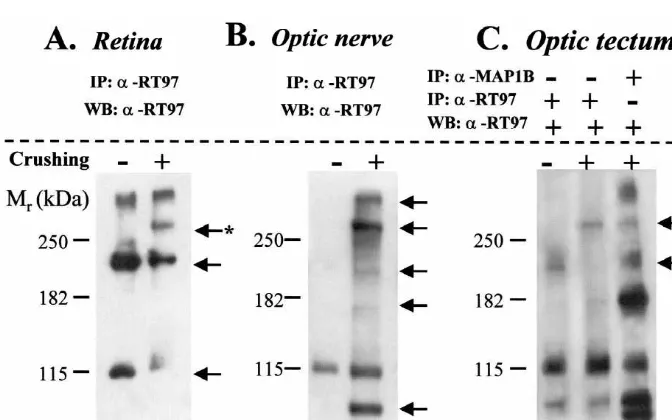
with Super Signal West Pico chemiluminescent substrate proteins as shown in Fig. 1A, lane 2, there appears to be an
(Pierce, USA). induction of the labeling of a band of approximately 325
kDa. There was no appreciable change in the labeling of
2.2. Digitally processed images the 335 kDa protein.
Images of processed tissues were obtained with an 3.1.2. Optic nerve
Olympus DP10 digital camera coupled to a Leica DMRB Proteins from nerve lysates were immunoprecipitated photomicroscope. Immunoblot-films were directly di- with anti-RT97 antibody followed by Western blot with the gitally-scanned. Original pictures were further processed same antibody. As shown in Fig. 1B, the pattern of optic with Adobe PhotoshopE 5.5 software so as to obtain the nerve proteins labeled in tench is dramatically altered after optimal contrast within the same figure plate. crushing. In control tench there is a modest labeling of a 115 kDa protein (lane 1). Optic nerve crushing resulted in a clear and marked increase in the labeling of this protein
3. Results (Fig. 1B, lane 2). Interestingly, there appears to be a
selective labeling of several other proteins in the optic
3.1. Immunoblotting /Western blot nerve induced after the lesion (105, 160, 200, 325 and 335
kDa, approximately). 3.1.1. Retina
Proteins from retina lysates were immunoprecipitated 3.1.3. Optic tectum
with anti-RT97 antibody followed by Western blot with the As seen in Fig. 1C (lane 1), the anti-RT97 recognized at same antibody. As shown in Fig. 1A, lane 1, the anti- least three proteins of different molecular weights in RT97, in normal conditions, clearly recognized at least control animals (105, 115 and 200 kDa approximately). three proteins of different molecular weights: 115, 200 and The optic nerve crushing (Fig. 1C, lane 2) did not modify 335 kDa approximately. The retina protein labeling pattern the labeling of the 105 and 115 kDa proteins appreciably, in tench was altered by optic nerve crushing. While there but induced a modest and selective labeling of a single was a decrease in the labeling of the 115 and 200 kDa high molecular weight protein (325 kDa, approximately).
The slight labeling of the band at approximately 200 kDa were seen to run along the more vitreal portion of the in control conditions disappeared after crushing the optic retina from the peripheral zone towards the optic nerve
nerve. head penetrating the central zone close to the central artery
(Fig. 2B).
3.1.4. Anti-MAP1B antibody In the control optic nerve, RT97-immunolabeling was
In order to study which proteins recognized by the RT97 weak and diffuse throughout the nerve, with the exception antibody are also recognized by the MAP1B antibody in of a small group of axons which were strongly labeled in the tench visual system, we performed an additional one of the nerve folds towards the interior part of the nerve experiment. We carried out an immunoprecipitation of (Fig. 3A).
proteins from the retina, nerve and optic tectum with the In the optic tectum (OT) RT97 labeled axons were MAP1B or RT97 antibodies followed by Western blot with located in the stratum marginale (SM), stratum opticum RT97 antibody. Only one representative result from tench (SO), the stratum fibrosum and griseum superficiale tissue, optic tectum, is shown in Fig. 1C, lane 3. This (SFGS) and stratum griseum centrale (SGC) (Fig. 5A). In experiment demonstrated that the RT97 antibody, in our the deeper zone of the SO and in the SFGS the immuno-conditions, could also recognize proteins purified with the reactive axons were organized in laminas whereas in the
MAP1B antibody in tench visual system. SGC the axons were isolated (Fig. 5A).
3.2. Immunohistochemistry 3.2.2. Lesioned animals
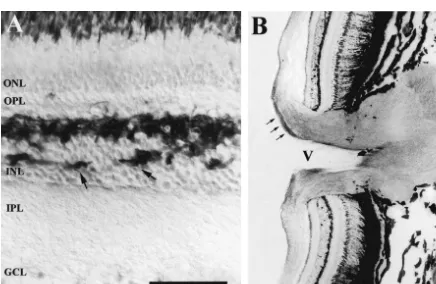
After optic nerve crush, we did not find modifications in
3.2.1. Control animals the labeling with RT97 in the retina.
In control retinas, the RT97 antibody labeled horizontal In the optic nerve,1 day after lesion the diffuse labeling cells and the growing ganglion cell axons that were being of the nerve disappeared in the area of the lesion, but was formed in the peripheral portion intensely (Fig. 2). The maintained in the rest of the nerve, both in the portion horizontal cells, located in the more vitreal level of the proximal to the eye and in the portion distal to the eye outer nuclear layer (ONL), showed immunoreactivity in toward the optic tract (Fig. 3B). In the first week after their perikarya and processes, which also penetrated the lesion diffuse staining was present in the area between the inner nuclear layer (Fig. 2A). The labeled growing axons retina and the zone of lesion, but disappeared completely
both in the lesion zone and in the rest of the nerve towards distribution of the labeling remained until 200 days post-the optic tract. At 15 days, in the proximal area of the lesion.
optic nerve the diffuse labeling was more intense while strongly labeled isolated axons were observed running from the retina towards the crush zone (Fig. 3C). Several
of these immunopositive axons crossed the damaged area, 4. Discussion
whereas in the rest of the nerve no labeling was observed.
One month after the lesion, the majority of the isolated In this study we describe the distribution of immuno-positive axons had crossed the damaged area and ran reactivity of RT97 antibody in the retina, optic nerve and toward the optic tract (Fig. 3D). In the lesioned zone the optic tectum of the normal tench and its modifications after labeling started to be diffuse. At 45 days after lesion, in optic nerve crush. We demonstrate, for the first time, that transverse sections of the optic nerve in the pre-lesion area the antibody RT97 is an excellent marker of growing and the labeling was observed to be diffuse (Fig. 3E). How- regenerated axons of the fish visual system. In normal ever, in the damaged area and in the area close to the animals, RT97 labels the growing ganglion cell axons lesion groups of axons presented different intensities of more intensely than the mature axons. These youngest labeling whereas the rest of the axons of the nerve were axons are continuously added in the peripheral retina of unlabeled (Fig. 3F). In the portion close to the retina and fish [26] and run from the more vitreal zone of the retina in the optic tract the labeling continued diffuse. At 90 towards the optic nerve head, penetrating the nerve sur-days, the group of axons labeled with RT97 increased rounding the central artery and run along the ventral zone notably crossing the lesion zone and reaching the distal of the optic nerve towards the optic tectum [11,18,24]. zone of the crush (Fig. 3G). At 120 days after crushing, Moreover, RT97 is an excellent marker for horizontal cells intensely labeled fibers were observed in the proximal and in the retina of tench, trout [6] and mammals [5]. In the lesioned areas. In the distal area a great number of isolated lesioned tench, the expression of RT97 increases notably axons were observed occupying the majority of the optic in the regenerating optic nerve axons in active growth. nerve both in its length and width (Fig. 4). Between 150 These results are similar to those obtained with other and 200 days after crushing the labeling of RT97 tended to neurofilament proteins such as plasticin both in normal and be similar to the normal optic nerve but several bundles of lesioned goldfish [12] which presents different labeling in
intensely labeled fibers remained. young and adult axons. However, RT97 is not detected in
In the optic tectum, 30 days after lesion, the RT97 the somata of ganglion cells.
labeled axons decreased notably in the SO and SFGS. A The pattern of spatial / temporal labeling with RT97 is few positive axons were observed in some levels of this parallel to that obtained using E587 antibody, which stratum (Fig. 5B). However, 90 days after crush, the specifically recognizes growing axons in the fish retinotec-intensely labeled axons started to increase in these strata tal pathway binding with a cell surface glycoprotein [35]. notably surpassing those of the control animals (Fig. 5C). In this study based on the tench visual system, we have At 120 days, the labeling in the OT occupied the same clearly demonstrated by Western blot analysis that the strata as in the normal animals, but in the SO dense groups RT97 antibody recognized, depending on the tissue ana-of intensely labeled axons appeared and in the SFGS lyzed and experimental conditions, at least 6 different positive axons were located at random (Fig. 5D). This protein bands, separated by 7.5% SDS–PAGE
phoresis (105, 115, 160, 200, 325 and 335 kDa, approxi- suitable for Western blot analysis in tench tissues in our experimental conditions. It has been demonstrated that mately).
MAP1B-phos, of 325 kDa, also undergoes modifications Western blot analysis demonstrated that the RT97
during the regeneration of the optic nerve of fish [34]. antibody labels different proteins in the normal tench
This approach of immunoprecipitation with MAP1B tissues. Moreover, this antibody recognizes several other
followed by Western blot with RT97 also showed that both proteins after optic nerve crushing principally in the optic
antibodies could recognize several other proteins in com-nerve. This indicates that during the regeneration process
mon. This observation could support several hypotheses: RT97 recognizes different proteins which are not detected
(i) MAP1B and RT97 antibodies could recognize the same in normal conditions. Furthermore, this is in accordance
epitope in tench proteins; in other words, they cross-react, with the variation of immunolabeling in the tissues
be-but to different degrees. It is worth mentioning that in the tween normal and lesioned animals in the optic nerve and
same conditions of lesion-regeneration, immunoprecipita-the optic tectum.
tion with MAP1B shows a very different pattern of The antibody RT97 has been used principally as a
labeling from RT97. Thus, for instance, the labeling of a marker for a mammal phosphorylated neurofilament
pro-325 kDa protein after immunoprecipitation with anti RT97 tein of 200 kDa and to a lesser degree one of 155 kDa [2],
induced by crushing is more intense and clear (Fig. 1C, tau and a developmental phosphorylated epitope of
lane 2) than the labeling observed after immunoprecipita-MAP1B [19]. In previous studies in which this antibody
tion with anti-MAP1B (Fig. 1C, lane 3). We could has been used in the visual system of rat [25,36] it has
conclude at this point that even if both antibodies cross-been stated that the axon labeling is due to the fact that this
react, they would show different affinities. (ii) When antibody recognizes a protein of the neurofilaments of high
immunoprecipitating, some non-related proteins, which are molecular weight (200 kDa). To ensure a sufficient amount
not recognized by the RT97 antibody, could be purified of protein in each analyzed tissue, we immunoprecipitated
due to stable protein–protein interactions that allowed their with RT97 prior to performing Western blot with the same
co-immunoprecipitation with those specific for the RT97 antibody. By using these two methods, we detected a
antibody. This technique of coimmunoprecipitation is protein with a molecular weight of 200 kDa, probably the
widely used and accepted to demonstrate protein–protein phosphorylated neurofilament protein. To try to confirm
interaction. this, we used a monoclonal Pan antibody to neurofilament
By using two different experimental techniques in this proteins which specifically reacts with all three major study we have demonstrated that the optic nerve of polypeptides of human neurofilaments (68, 160 and 200 lesioned tench presents a considerable increase of global kDa). Unfortunately our Western blot experiments indicate immunoreactivity to anti-RT97. This is due to an inten-that this antibody does not function adequately in tench, in sified and induced labeling of several proteins. In conclu-comparison to a positive control from rat, which showed sion, we can affirm that RT97 is a good marker for the three neurofilament polypeptides. We cannot draw any growing and regenerating axons in the visual system of conclusions from this Pan antibody in tench tissues, at tench and that this antibody immunoreacts with several least using a Western blot approach. More exhaustive proteins in normal and regeneration conditions. Further biochemical studies would be necessary to resolve whether work would be necessary to identify those RT97-labeled the 200 kDa tench protein labeled with RT97 corresponds proteins that present an increased or induced
immuno-to a neurofilament protein. reactivity after lesion or during regeneration of the tench
Although the proteins of the neurofilaments of the optic optic nerve. nerve of fish, both in the normal state and in regeneration,
are found in the low and medium ranges of molecular
weights and the existence of NF-H proteins has not been Acknowledgements demonstrated [30], the appearance of a band of 200 kDa
labeled with the antibody RT97 in the regenerated axons at We would like to thank Dr. Medina and Dr. A. 30 days post-lesion (Fig. 1B, lane 2) would indicate that Tabernero for great technical collaboration with Western there could be an activation of phosphorylated proteins of blot and Mr. G.H. Jenkins for revising the English version
high molecular weight. of the ms. The monoclonal antibody RT97 developed by J.
With regard to proteins of high molecular weight that Wood was obtained from the Developmental Studies are recognized with the antibody RT97, we showed, by Hybridoma Bank maintained by the University of Iowa. immunoprecipitation with MAP1B or RT97 antibodies This work was supported by the Junta de Castilla y Leon´ followed by Western Blot with RT97 antibody, that anti- (SA23 / 99), and Spanish DGICYT (PB97-1341) projects. RT97 identifies two bands of greater than 250 kDa.
Probably these two proteins represent both phosphorylated
isoforms of MAP1B, of 335 and 325 kDa (Fig. 1C, lane References 3). We were unable to show that both bands,
immuno-precipitated with MAP1B antibody, correspond, in fact, to [1] L. Alfei, A. Onali, B. Caronti, A.M. Valente, L. Medolago Albani, C.
neurofilament epitopes are co-distributed in the fish Mauthner axon, [20] Y. Kitao, B. Robertson, M. Kudo, G. Grant, Neurogenesis of Cell Mol. Biol. 44 (1998) 605–614. subpopulations of rat lumbar dorsal root ganglion neurons including [2] B.H. Anderton, D. Breinburg, M.J. Downes, P.J. Green, B.E. neurons projecting to the dorsal column nuclei, J. Comp. Neurol.
Tomlinson, J. Ulrich, J.M. Wood, J. Kahn, Monoclonal antibodies 371 (1996) 249–257.
show that neurofibrillary tangles and neurofilaments share antigenic [21] H. Ksiezak-Reding, D.W. Dickson, P. Davies, S.H. Yen, Recognition determinants, Nature 298 (1982) 84–86. of tau epitopes by anti-neurofilament antibodies that bind to [3] B. Anderton, H.B. Coakham, J.A. Garson, A.A. Harper, E.I. Harper, Alzheimer neurofibrillary tangles, Proc. Natl. Acad. Sci. USA 84
S.N. Lawson, A monoclonal antibody against neurofilament protein (1987) 3410–3414.
specifically labels the large light cell population in rat dorsal root [22] D.D. Larrivee, B. Grafstein, Phosphorylation of proteins in normal ganglia, J. Physiol. (Lond.) 334 (1983) 97–98P. and regenerating goldfish optic nerve, J. Neurochem. 49 (1987) [4] D.G. Attardi, R.W. Sperry, Preferential selection of central pathways 1747–1757.
by regenerating optic fibers, Exp. Neurol. 7 (1963) 46–64. [23] S.N. Lawson, A.A. Harper, E.I. Harper, J.A. Garson, B.H. Anderton, ¨
[5] G.W. Balkema, U.C. Drager, Light-dependent antibody labeling of A monoclonal antibody against neurofilament protein specifically photoreceptors, Nature 316 (1985) 630–633. labels a subpopulation of rat sensory neurones, J. Comp. Neurol. [6] H.M. Blank, B. Muller, H. Korf, Comparative investigations of the 228 (1984) 263–272.
´
neuronal apparatus in the pineal organ and retina of the rainbow [24] C. Lillo, A. Velasco, D. Jimeno, J.M. Lara, J. Aijon, Ultrastructural trout: immunocytochemical demonstration of neurofilament 200-kDa organization of the optic nerve of the tench (Cyprinidae, Teleostei), and neuropeptide Y, and tracing with DiI, Cell Tissue Res. 288 J. Neurocytol. 27 (1998) 593–604.
(1997) 417–425. [25] L. McKerracher, M. Vidal-Sanz, A.J. Aguayo, Slow transport rates [7] M.J. Bragado, G.E. Groblewski, J.A. Williamas. p70s60k is acti- of cytoskeletal proteins change during regeneration of axotomized
vated by CCK in rat pancreatic acini. Am. J Physiol. 273 (1997) retinal neurons in adult rats, J. Neurosci. 10 (1990) 641–648. ¨
(Cell Physiol. 42) C101–106. [26] H. Muller, Bau und Wachstum der Netzhaut des Guppy (Lebistes [8] M.P. Coleman, B.H. Anderton, Phosphate-dependent monoclonal reticulatus), Zool. Jb. Abt. Allgemeine Zool. Physiol. 63 (1952)
antibodies to neurofilaments and Alzheimer neurofibrillary tangles 275–324.
recognize a synthetic phosphopeptide, J. Neurochem. 54 (1990) [27] W. Quitschke, N. Schechter, In vitro protein synthesis in the goldfish 1548–1555. retinotectal pathway during regeneration: Evidence for specific [9] D. Dahl, C.J. Crosby, A. Bignami, Neurofilament proteins in fish: a axonal proteins of retinal origin in the optic nerve, J. Neurochem. 41
study with monoclonal antibodies reacting with mammalian NF 150 (1983) 1137–1142.
K and NF 200 K, J. Comp. Neurol. 250 (1986) 399–402. [28] W. Quitschke, N. Schechter, Specific optic nerve proteins during [10] D. Dahl, C.J. Crosby, E.E. Gardner, A. Bignami, Delayed phos- regeneration of the goldfish retinotectal pathway, Brain Res. 258
phorylation of the largest neurofilament protein in rat optic nerve (1983) 69–78.
development, J. Neurosci. Res. 15 (1986) 513–519. [29] W. Quitschke, N. Schechter, At 58 000 dalton intermediate filament [11] S.S. Easter, A.C. Rusolff, P.E. Kish, The growth and organization of proteins of neuronal and non-neuronal origin in the goldfish visual
the optic nerve and tract in juvenile and adult goldfish, J. Neurosci. pathway, J. Neurochem. 42 (1984) 569–576.
1 (1981) 793–811. [30] W. Quitschke, P.S. Jones, N. Schechter, A survey of intermediate [12] C. Fuchs, E. Glasgow, P.F. Hitchcock, N. Schechter, Plasticin, a filament proteins in optic nerve and spinal cord: evidence for
newly identified neurofilament protein, is preferentially expressed in differential expression, J. Neurochem. 44 (1985) 1465–1476. young retinal ganglion cells of adult goldfish, J. Comp. Neurol. 350 [31] R.W. Sperry, Patterning of central synapses in regeneration of the (1994) 452–462. optic nerve in teleosts, Physiol Zool. 21 (1948) 351–361. [13] S. Giordano, E. Glasgow, R. Druger, N. Schechter, Intermediate [32] P.M. Steinert, D.R. Roop, Molecular and cellular biology of
filaments. A molecular link to nerve development and regeneration intermediate filaments, Annu. Rev. Biochem. 57 (1988) 593–625. in the goldfish visual pathway, in: A. Vernadakis, B.I. Roots (Eds.), [33] P. Tesser, P.S. Jones, N. Schechter, Elevated levels of retinal Neuron-glia Interrelations During Phylogeny. II. Plasticity and neurofilament mRNA accompany optic nerve regeneration, J. Regeneration, Humana Press Inc, Totowa, NJ, 1995, pp. 367–389. Neurochem. 47 (1986) 1235–1243.
[14] E. Glasgow, R.K. Druger, E.M. Levine, C. Fuchs, N. Schechter, [34] E. Vecino, L. Ulloa, J. Avila, The phosphorylated isoform of Plasticin, a novel type III neurofilament protein from goldfish retina: microtubule associated protein (MAP1B) is expressed in the visual increased expression during optic nerve regeneration, Neuron 9 system of tench (Tinca tinca L.) during optic nerve regeneration,
(1992) 373–381. Neurosci. Lett. 245 (1998) 93–96.
[15] E. Glasgow, R.K. Druger, C. Fuchs, W.S. Lane, N. Schechter, [35] J. Vielmetter, F. Lottspeich, C.A.O. Stuermer, The monoclonal Molecular cloning of gefiltin (ON1): serial expression of two new antibody E587 recognizes growing (new and regenerating) retinal neurofilament mRNAs during optic nerve regeneration, EMBO J. 13 axons in the goldfish retinotectal pathway, J. Neurosci. 11 (1991)
(1994) 297–305. 3581–3593.
´
[16] E. Glasgow, C.M. Hall, N. Schechter, Organization, sequence, and [36] M.P. Villegas-Perez, J.M. Lawrence, M. Vidal-Sanz, M.M. Lavail, expression of a gene encoding goldfish neurofilament medium R.D. Lund, Ganglion cell loss in RCS rat retina: a result of protein, J. Neurochem. 63 (1994) 52–61. compression of axons by contracting intraretinal vessels linked to [17] B. Grafstein, The retina as a regenerating organ, in: R. Adler, D. the pigment epithelium, J. Comp. Neurol. 392 (1998) 58–77.
Ferher (Eds.), The Retina: A Model For Cell Biology Studies, [37] J.N. Wood, B.H. Anderton, Monoclonal antibodies to mammalian Academic Press Inc, New York, 1986, pp. 275–335. neurofilaments, Biosci. Rep. 1 (1981) 263–268.
[18] P.A.R. Johns, Growth of adult goldfish eye. III: Source of the new [38] Y. Zhao, B.G. Szaro, The return of phosphorylated and non-phos-retinal cells, J. Comp. Neurol. 176 (1977) 343–358. phorylated epitopes of neurofilament proteins to the regenerating [19] M. Johnstone, R.G. Goold, I. Fischer, P.R. Gordon-Weeks, The optic nerve of Xenopus laevis, J. Comp. Neurol. 343 (1994) 58–
neurofilament antibody RT97 recognizes a developmentally reg- 172. ulated phosphorylation epitope on microtubule-associated protein