Aspartate metabolism in
Cicer
immature seeds requires Ca
2+,
protein phosphorylation and dephosphorylation
Madhusudan Dey, Sipra Guha-Mukherjee *
School of Life Sciences,Jawaharlal Nehru Uni6ersity,New Delhi110067,India
Received 27 August 1998; accepted 7 September 1999
Abstract
The enzyme, aspartate kinase (AK) which is regulated through feedback mechanism by its end products, lysine and threonine, controls aspartate metabolism in plants. Here evidence is provided that intracellular signalling molecules such as Ca2+, protein
kinases and phosphatases also participate in regulating AK activity in immature seeds ofCicer. Elevated levels of intracellular Ca2+ activate AK, which is mediated through protein phosphorylation cascade. © 2000 Elsevier Science Ireland Ltd. All rights
reserved.
Keywords:Aspartate kinase; Signalling molecules; Protein kinase; Phosphatase
www.elsevier.com/locate/plantsci
1. Introduction
Amino acid metabolism in plants is controlled by a complex regulatory network involving a num-ber of enzymes, intermediates etc. [1]. Aspartate kinase (AK, EC 2.7.2.4), the key enzyme in the branched chain amino acid metabolic pathway, catalyzes the first reaction converting aspartate to b-aspartyl phosphate. The existence of two or three AK isozymes have been identified in plants. In barley, there are three isozymes: one of which is sensitive to threonine while the other two to are sensitive to lysine [2]. Likewise in carrot one AK isozyme is sensitive to threonine and the other one to lysine [3]. Three AK isozymes have been re-ported in maize [4,5] and different cDNA clones have been characterized [6]. It has been shown that some AK isozymes appear to be bifunctional proteins that carry out the catalytic activity of both AK and homoserine dehydrogenase [3,5,7]. Tang et al. [8] also reported the existence of mono-functional AK in Arabidopsis homologous to
lysine-sensitive enzyme ofEscherichia coli. Regula-tion of aspartate family pathway has been re-viewed in detail by Galili [1] and Singh [9]
The aspartate metabolism in bacteria is regu-lated by two mechanisms: (1) feedback inhibition of AK by its end products; and (2) repression of gene encoding AK [10]. Although the former mechanism is well established in plants, the latter is not [1,11]. Besides, Giovanelli [12] showed that lysine-sensitive isozyme of AK in Lemna pauci-costatawas synergistically inhibited by S-adenosyl-methionine (SAM) at low concentration of lysine whereas SAM itself had no effect on AK activity. Various intra-cellular molecules such as Ca2+,
cyclic AMP, 1,4,5-inositol triphosphate (IP3),
protein kinases and phosphatases are known to orchestrate the signalling cascade in plants [13 – 16]. The activation or inhibition of various metabolic and catabolic pathways is also mediated through this cascade [17 – 19].
It is well established that Ca2+ plays an
impor-tant role in plant signal trafficking. The regulatory action of Ca2+ ranges from control of ion
trans-port to gene expression. A rapid and transient increase in cytosolic Ca2+ concentration upon
dif-* Corresponding author. Tel.:+91-11-618-7175; fax:+ 91-11-616-9962.
E-mail address:[email protected] (S. Guha-Mukherjee)
ferent extra-cellular stimuli is considered as the primary event in different physiological responses [20 – 22] and in activation of different biochemi-cal pathways [23]. In earlier studies it was shown that phytochrome activation of AK is mediated through Ca2+ [24]. Here evidence is provided
that elevated level of intracellular Ca2+ in
im-mature seeds activates AK. Moreover, protein kinase and phosphatase inhibitors reduce the stimulatory effect of Ca2+. Therefore it appears
that the elevated level of cytosolic Ca2+
concen-tration, protein phosphorylation and dephospho-rylation are necessary for activating AK. Another unrelated protein glutathione S-trans-ferase (GST), which is involved in detoxification of several herbicides like chloroacetanilides, thio-carbamates, s-triazines [25], is taken as an inter-nal control to account for the specificity of the calcium effect.
2. Materials and methods
2.1. Plant material
Seeds of Cicer arietinum var Pusa362 were ob-tained from Indian Agricultural Research Insti-tute, New Delhi. All experiments were performed with plants grown in the field during winter.
2.2. Chemical treatments to de6eloping pods of
Cicer
Developing pods of Cicer were treated with different chemicals when the pod size was ap-proximately 10 mm×5 mm×5 mm. Pods, which were injected with Ca2+, Mg2+
ethyleneg-lycol-bis-(b-aminoethyl ether) N%,N%,N%,N%, -te-traacetic acid) (EGTA), verapamil, ionomycin, H7 (1 - (5 - isoquinolinylsulfonyl) - 2 - methylpiper-azine) and sodium vanadate in the morning were harvested after 24 h and stored at −80°C. All chemicals were prepared at the required concen-trations and calibrated at pH 7.0 except for H7 and EGTA which were at pH 8.0. Pods injected with distilled water were used as control. The concentrations of various chemicals used are given in the respective legends. All chemicals were obtained from ‘Sigma’ except sodium vana-date, which was from ‘Fluka’.
2.3. Enzyme extraction and partial purification
Seeds excised from treated pods were homoge-nized in a mortar and pestle in 100 mM Tris – HCl pH 7.5, containing 50 mM KCl, 1 mM DTT (dithiothreitol), 0.1 mM phenylmethylsulfonylfluo-ride (PMSF), 20% ethanediol and 5% insoluble polyvinylpyrrolidone. The homogenate was centrifuged for 30 min at 20 000×g and the supernatant was subjected to 30% ammonium sulfate saturation. Supernatant was centrifuged at the same speed and was further subjected to 55% ammonium sulfate saturation. The precipitate was dissolved in 50 mM Tris – HCl pH 7.5 containing 50 mM KCl, 1 mM DTT and 10% ethanediol and dialyzed at 4°C for 6 h against excess of the same buffer. This 30 – 55% fraction of enzyme was used for all assays.
2.4. AK assay and protein estimation
AK was assayed by modified aspartyl hydroxamate method [26]. The assay mixture contains 100 mM Tris – HCl pH 8.0, 50 mM hydroxyamyl HCl pH 8.0, 20 mM Na2ATP, 100
mM aspartate and 40 mM MgSO4. The reaction
carried out at 37°C for 60 min was terminated by addition of 500ml of 0.67 M FeCl3, containing 0.37
M HCl and 20% TCA. After removal of protein precipitate by centrifugation the absorbance of supernatant was read at 505 nm. The protein concentration was determined following the procedure of Bradford [27]. One unit of enzyme activity (IU) is defined as the amount AK catalyzing the production of 1 mmol of b-aspartylphosphate h−1 at 37°C.
2.5. GST assay
(extinction coefficient at 340 nm=9.6 mM−1
cm−1). The specific activity of enzyme is defined
as units of enzyme mg protein−1.
3. Results
3.1. Acti6ation of AK by calcium
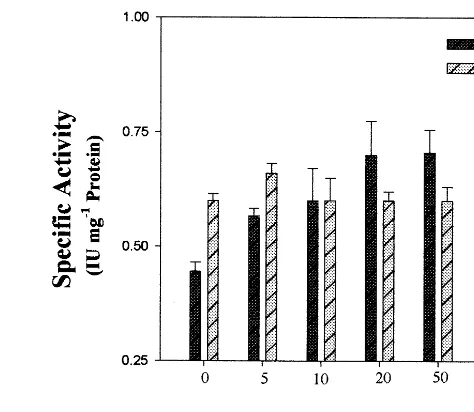
There are several lines of evidence which suggest that an elevated level of Ca2+ concentration in the
cytosol is responsible for the varying responses encountered with different signals, such as illumi-nation [22], stress [28] and hormones [29]. In order to investigate the role of intracellular Ca2+ in
activating the AK increasing concentrations of Ca2+ was injected into the developing pods of
Cicerand their effects were analyzed. Fig. 1 shows that increasing concentrations of Ca2+ (upto 20
mM) stimulated AK activity. In contrast, injection of Mg2+ into the developing pods of Cicer at
different concentrations had no significant enhanc-ing effect (data not shown). Plant GSTs are in-duced either by biotic factors such as pathogen invasion or abiotic factors such as herbicide safen-ers and heavy metals [30]. Here GST was used as an internal control to account for the specificity of the calcium effect. No significant change of GST activity with Ca2+ treatment was observed (Fig.
1).
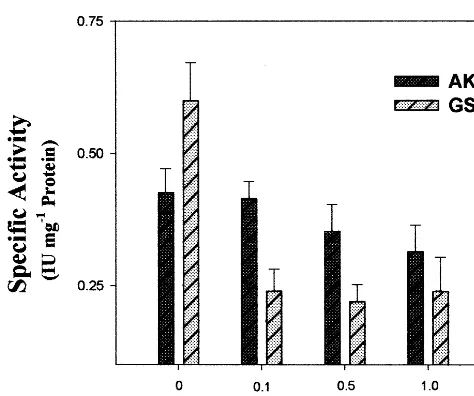
Fig. 2. Effect of ethyleneglycol-bis-(b-aminoethyl ether) N%,N%,N%,N%,-tetraacetic acid) (EGTA) on in vivo aspartate
kinase (AK) and glutathione S-transferase (GST) specific activity. EGTA was injected into the developing pods at different concentrations as indicated in the histogram. Each value represents the mean (S.E. of three experiments).
To confirm the possible involvement of Ca2+ in
activation of AK we injected a calcium chelator, EGTA at different concentrations into the devel-oping pods of Cicer. It was found that 10 mM EGTA significantly reduced the basal level of AK activity whereas GST remained the same (Fig. 2). Moreover, it was observed that the injection of 20 mM Ca2+ along with 10 mM EGTA reduced the
Ca2+ effect by 32% (Fig. 3).
3.2. Role of calcium channel blocker and calcium ionophore
To test further the enhancing role of Ca2+
verapamil was employed which has been reported to block Ca2+ channels localized in the plasma
membrane [21]. A significant decrease in AK activ-ity was noted with 1 – 10 mM concentrations of verapamil (Fig. 4). Injection of 5 mM verapamil 2 h before the Ca2+ treatment reduced the Ca2+
effect significantly (Fig. 3). But this channel blocker did not have any significant effect on GST activity.
Ionomycin, which has been reported to increase the intracellular Ca2+ concentration [31], was
in-jected into the developing pods ofCicer. Although there was a progressive stimulation of AK activity, no significant enhancing effect was noted for GST (Fig. 5).
Fig. 1. Effect of Ca2+ on in vivo aspartate kinase (AK) and
glutathione S-transferase (GST) specific activity. Ca2+ was
Fig. 3. Combined effects of Ca2+ and ethyleneglycol-bis-(b
-aminoethyl ether) N%,N%,N%,N%, -tetraacetic acid) (EGTA),
verapamil, H7 or vanadate on in vivo aspartate kinase (AK) and glutathione S-transferase (GST) activity. Developing pods were injected with 10 mM EGTA, 5 mM verapamil, 0.2 mM H7, 1 mM vanadate, and 2 h later with 20 mM Ca2+.
Pods were harvested after 24 h, immature seeds were excised and analyzed for AK and GST activity. Each value represents the mean (S.E. of three experiments).
Fig. 5. Effect of ionomycin on in vivo aspartate kinase (AK) and glutathione S-transferase (GST) specific activity. Iono-mycin was injected into the developing pods at different concentrations as indicated in the histogram. Each value represents the mean (S.E. of three experiments).
to examine the possible role of these protein ki-nases and phosphatases on AK activation, two separate experiments were performed. In the first experiment, H7, a potent protein kinase inhibitor [33] was injected at different concentrations (0.1 – 0.5 mM) into the developing pods of Cicer and it was observed that there was a sharp decrease in 3.3. Effect of protein kinase and phosphatase
inhibitor
In all eukaryotic organisms intra-cellular sig-nalling cascades are mediated through different protein kinases and phosphatases [16,32]. In order
Fig. 6. Effect of protein kinase inhibitor, H7 on in vivo aspartate kinase (AK) and glutathione S-transferase (GST) specific activity. H7 was injected into the developing pods at different concentrations as indicated in the histogram. Each value represents the mean (S.E. of three experiments). Fig. 4. Effect of verapamil on in vivo aspartate kinase (AK)
Fig. 7. Effect of protein phosphatase inhibitor, vanadate on in vivo aspartate kinase (AK) and glutathione S-transferase (GST) specific activity. Vanadate was injected into the devel-oping pods at different concentrations as indicated in the histogram. Each value represents the mean (S.E. of three experiments).
nificant roles in activating AK, the key enzyme in the branched chain amino acid biosynthetic pathway.
The immature seeds in the developing pods, considered to be physiologically active organs where all metabolic and catabolic pathways oper-ate simultaneously, showed maximum AK activity with threonine-sensitive isozyme being the pre-dominant form. Therefore, it represents an excel-lent system for the study of signalling cascades controlling metabolic processes. Injections of Ca2+ upto 20 mM into the developing pods
changed its internal level, and thereby altered the physiological environment. Consequently, alter-ation took place in several biochemical pathways by the modulation of various enzymes like AK. The Ca2+-mediated activation of AK in vivo
pos-sibly occurred through the activation of a chain of other factors since Ca2+ did not have any direct
effect on AK activity in vitro [38]. Moreover, Ca2+ induced enhancement was not due to its
cationic effect as injection of Mg2+ did not change
the AK activity.
Injection of different concentrations of EGTA (a Ca2+ chelator) and verapamil (a channel
blocker) showed that these compounds had signifi-cant inhibitory effects on AK activity at 10 and 5 mM concentrations, respectively. Ionomycin, on the other hand, had stimulated AK activity. These observations establish that Ca2+ plays an active
role in regulating aspartate metabolism.
Generally, in vivo signalling cascades in plants are mediated through Ca2+, diacyl glycerol,
inosi-tol triphosphate (IP3) and protein phosphorylation
and dephosphorylation [39 – 41]. Protein kinase in-hibitor (H7) and phosphatase inin-hibitor (vanadate) were injected in immature seeds at various concen-trations in order to assess the possible involvement of protein phosphorylation and dephosphorylation in regulating AK activity. Both these compounds H7 and vanadate, individually inhibited the basal level of AK activity in vivo, by 71 and by 26% at 0.5 and 1 mM concentrations, respectively. Be-sides, they significantly reduced the stimulatory effect of Ca2+ (Fig. 3). This shows that both
kinases and phosphatases are required to activate AK. Vanadate was found to inhibit the GST activity as well, for reasons hitherto unknown. Interestingly, addition of these inhibitors did not change the AK activity in vitro (data not shown). In cascade signalling the kinases and phosphatases AK specific activity (Fig. 6). Similarly with
injection of vanadate, a protein phosphatase inhibitor [34] was injected and a gradual decrease in enzyme activity was observed with the increase in vanadate concentrations (Fig. 7). These results suggest involvement of both protein kinases and phosphatases on activation of AK. GST activity which was not influenced by H7 treatment was significantly inhibited by vanadate (Figs. 6 and 7). This could be due to the involvement of phosphatase 2B type in regulating GST. Furthermore, an injection of 0.2 mM H7 or 1 mM vanadate 2 h before 20 mM Ca2+ treatment
significantly reduced the enhancing effect of Ca2+
by 71 and 57%, respectively (Fig. 3).
4. Discussion
sig-act in reverse of each other while sig-activating cer-tain phenomenon. However, the requirement of both kinases and phosphatases have also been reported to be essential for the activation of M-phase-promoting factor (MPF) during eukaryotic cell division, which is regulated by both phos-phorylation and dephosphos-phorylation. In MPF, two protein kinases are involved in phosphory-lating two sites in cdc 2 protein, which still re-mains inactive until one phosphate is removed by the phosphatase cdc 25 [42,43]. It is well es-tablished that protein kinase/phosphatase gener-ally change the conformation of protein to give its active/inactive form. Therefore we suggest that either mechanism like MPF or some inter-nal mediators, not known yet, are part of this signalling cascade, which require the presence of Ca2+, protein phosphorylation and
dephospho-rylation for activating aspartate metabolism. The observations, mainly focused on the acti-vation of total activity of AK, showed that threonine-sensitive form of AK is predominant in immature seed of Cicer. Although these stud-ies do not reveal the accurate mechanism, it pro-vides an insight into how the signalling cascades occur inside the cell to modulate the aspartate metabolism, which has not been reported yet. Therefore, it was suggested that the entry of 4-carbon unit by AK into the aspartate family biosynthetic pathway is not only inhibited by feedback mechanism, but also significantly influ-enced by other signalling molecules such as Ca2+, protein kinase and phosphatases.
Molecu-lar cloning of isozyme specific gene and its de-velopmental regulation in particular will further reveal its functional significance.
Acknowledgements
This work was supported by grants from the Department of Biotechnology, Government of India to the National Centre for Plant Genome Research. MD is grateful to the UGC for a Ju-nior Research Fellowship.
References
[1] G. Galili, Regulation of lysine and threonine synthesis, Plant Cell 7 (1995) 899 – 906.
[2] S.E. Rognes, S.W.J. Bright, B.J. Miflin, Feed back-insen-sitive aspartate kinase isozymes in barley mutants resis-tant to lysine plus threonine, Planta 157 (1983) 32 – 38. [3] J.M. Weismann, B.F. Matthews, Identification and
ex-pression of a cDNA from Daucus carota encoding a bifunctional aspartokinase-homoserine dehydrogenase, Plant Mol. Biol. 22 (1993) 301 – 312.
[4] S.B. Dotson, D.A. Somers, B.G. Gengenbach, Purifica-tion and characterizaPurifica-tion of lysine-sensitive aspartate kinase in maize cell culture, Plant Physiol. 91 (1989) 1602 – 1608.
[5] R.A. Azevedo, R.D. Blackwell, R.J. Smith, P.J. Lea, Three aspartate kinase isozymes from maize, Phytochem-istry 31 (1992) 3725 – 3730.
[6] J.M. Gray, A.S. David, F.M. Benjamin, G.G. Burley, Molecular genetics of the maize (Zea maysL.) aspartate kinase-homoserine dehydrogenase gene family, Plant Physiol. 106 (1994) 1303 – 1312.
[7] M. Ghislain, V. Frankard, D. Vandenbossche, B.-F. Matthews, M. Jacobs, Molecular analysis of aspartate kinase-homoserine dehydrogenase gene fromArabidopsis thaliana, Plant Mol. Biol. 24 (1994) 835 – 851.
[8] G. Tang, J.X. Zhu-Shimini, R. Amir, I.B. Zchori, G. Galili, Cloning and expression of anArabidopsis thaliana cDNA encoding a monofunctional aspartate kinase ho-mologous to the lysine-sensitive enzyme of Escherichia coli, Plant Mol. Biol. 34 (1997) 287 – 293.
[9] B.K. Singh, D.L. Shaner, Biosynthesis of branched chain amino acid: from test tube to field, Plant Cell 7 (1995) 935 – 944.
[10] G.N. Cohen, I. Saint-Girons, Biosynthesis of threonine, lysine and methionine, in: F.C. Neidhardt (Ed.), Es -cherichia coli andSalmonella typhimurium. Cellular and Molecular Biology, American Society for Microbiology, Washington, DC, 1987, pp. 429 – 444.
[11] S.J. John, S. Guha-Mukherjee, in: K.K. Tiwari, G.S. Singhal (Eds.), Plant Molecular Biology and Biotechnol-ogy, Narosa, New Delhi, 1997, pp. 17 – 18.
[12] J. Giovanelli, S. Mudd, A. Datko, In vivo regulation of threonine and isoleucine biosynthesis in Lemna pauci -costataHegelm 6746, Plant Physiol. 86 (1988) 369 – 377. [13] Y. Nishizuka, Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C, Science 258 (1992) 607 – 614.
[14] K. Takahashi, M. Isobe, S. Muto, Mastoparan induces an increase in cytosolic calcium ion concentration and subsequent activation of protein kinase in tobacco sus-pension culture cells, Biochim. Biophys. Acta 1401 (1998) 339 – 346.
[15] J. Sheen, Ca2+-dependent protein kinases and stress
signal transduction in plant, Science 274 (1996) 1900 – 1902.
[16] P. Lessard, M. Kresis, M. Thomas, Protein phosphatases and protein kinases in higher plants, C. R. Acad. Sci. III 320 (1997) 675 – 688.
[18] H.G. Nimmo, P.J. Canter, P.A. Fewson, G.A. Mc Naughton, G.A. Nimmo, M.B. Wilkins, Regulation of phosphoenolpyruvate carboxylase: an example of signal transduction via protein phosphorylation in higher plants, Adv. Enzyme Regul. 30 (1990) 121 – 131. [19] N. Bakrim, C. Echevarria, C. Cretin, M. Arrio-Dupont,
J.N. Pierre, J. Vidal, R. Chollet, P. Gadal, Regulatory phosphorylation of sorghum leaf phosphoenolpyruvate carboxylase, identification of the protein serine-kinase and some elements of the signal transduction, Eur. J. Biochem. 204 (1992) 821 – 830.
[20] M.R. Knight, A.K. Campbell, S.M. Smith, A.J. Tre-wavas, Transgenic plant aequorin reports the effect of touch and cold shock and elicitor on cytosolic calcium, Nature 352 (1991) 524 – 526.
[21] M.R. Knight, S.M. Smith, A.J. Trewavas, Wind induced plant motion immediately increases cytosolic calcium, Proc. Natl. Acad. Sci. USA 89 (1992) 4967 – 4971. [22] P.S. Shacklock, N.D. Read, A.J. Trewavas, Cytosolic
free calcium mediates red light-induced photomorpho-genesis, Nature 358 (1992) 753 – 755.
[23] H. Karchi, D. Miron, S. Ben-Yaacov, G. Galili, The lysine-dependent stimulation of lysine catabolism in to-bacco seed requires Ca2+ and protein phosphorylation,
Plant Cell 7 (1995) 1963 – 1970.
[24] M. Dey, S. Guha-Mukherje, Phytochrome activation of aspartate kinase in etiolated chickpea (Cicer arietinum) seedlings, J. Plant Physiol. 154 (1998) 454 – 458.
[25] J.W. Gronwald, K.L. Plaisance, Isolation and characteri-zation of glutathione S-transferase isozymes from sor-ghum, Plant Physiol. 117 (1998) 877 – 892.
[26] B.J. Wilson, A.C. Gray, B.F. Matthews, Bifunctional protein in carrot contains both aspartokinase and ho-moserine dehydrogenase activities, Plant Physiol. 97 (1991) 1323 – 1328.
[27] M.M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding, Anal. Biochem. 72 (1976) 248 – 254.
[28] H. Knight, A.J. Trewavas, M.R. Knight, Calcium signal-ing in Arabidopsis thaliana responding to drought and salinity, Plant J. 12 (1997) 1067 – 1078.
[29] D.S. Bush, Calcium regulation in plant cells and its role in signaling, Annu. Rev. Plant Physiol. Plant Mol. Biol. 46 (1995) 95 – 122.
[30] K.A. Marrs, The function and regulation of glutathion S-transferase in plants, Annu. Rev. Plant Physiol. Plant Mol. Biol. 47 (1996) 127 – 158.
[31] C.-M. Liu, T.E. Hermann, Characterization of iono-mycin as a calcium ionophore, J. Biol. Chem. 253 (1978) 5892 – 5894.
[32] D.E. Clapham, Calcium signaling, Cell 80 (1995) 259 – 268.
[33] H. Hikada, M. Inagaki, S. Kawamoto, Y. Sasaki, Iso-quinolinesulfonamides; novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C, Biochemistry 23 (1984) 5036 – 5041.
[34] K. Harter, S. Kircher, H. Frohnmeyer, M. Krenz, F. Nagy, E. Schafer, Light regulated modification and nu-clear translocation of cytosolic G-Box binding factors in parsley, Plant Cell 6 (1994) 545 – 559.
[35] O. Shaul, G. Galili, Threonine overproduction in trans-genic tobacco plants expressing a mutant desensitized aspartate kinase of Escherichia coli, Plant Physiol. 100 (1992) 1157 – 1163.
[36] H. Karchi, O. Shaul, G. Galili, Seed specific expression of a bacterial desensitized aspartate kinase increases the production of seed threonine and methionine in trans-genic tobacco, Plant J. 3 (1993) 721 – 727.
[37] J.X. Zu-Shimoni, G. Galili, Expression of anArabidopsis aspartate kinase/homoserine dehydrogenase gene is metabolically regulated by photosynthesis-related signals but not by nitrogenous compounds, Plant Physiol. 116 (1998) 1023 – 1028.
[38] P.L.R. Bonner, A. Hetherington, P.J. Lea, Plant aspar-tate kinase is not activated by calmodulin or calcium, in: A.J. Trewaves (Ed.), Molecular and Cellular Aspects of Calcium in Plant Development, Plenum Press, New York, 1986, pp. 369 – 370.
[39] D.M. Roberts, A.C. Harmon, Calcium modulated proteins: targets of intracellular calcium signals in higher plants, Annu. Rev. Plant Physiol. Plant Mol. Biol. 43 (1992) 375 – 414.
[40] C. Despres, R. Subramaniam, D.P. Matton, N. Brisson, The activation of the potato PR-10a gene requires the phosphorylation of the nuclear factor PBF-1, Plant Cell 7 (1995) 589 – 598.
[41] S.-H. Kwak, S.H. Lee, The requirements of Ca2+,
protein phosphorylation and dephosphorylation for ethylene signal transduction in Pisum sati6um L., Plant Cell Physiol. 38 (1997) 1142 – 1149.
[42] D. Fesquet, J.C. Labbe, J. Derancourt, J.P. Capony, S. Galas, F. Girard, T. Lorca, J. Shuttleworth, M. Doree, J.C. Cavadore, The MO15 gene encodes the catalytic subunit of a protein kinase that activates cdc2 and other cyclin-dependent kinases (CDKs) through phosphoryla-tion of Thr161 and its homologoues, EMBO J. 12 (1993) 3111 – 3121.
[43] A. Kumagai, W.G. Dunphy, Regulation of the cdd25 protein during cell cycle in Xenopous extracts, Cell 70 (1992) 139 – 151.