Consequences on ethylene metabolism of inactivating the
ethylene receptor sites in diseased non-climacteric fruit
E.D. Mullins * , T.G. McCollum, R.E. McDonald
U.S.Horticultural Research Laboratory,Agricultural Research Ser6ice,U.S.Department of Agriculture,Ft Pierce,FL34945,USA Received 9 June 1999; accepted 16 January 2000
Abstract
Penicillium digitatum-infected grapefruit synthesize large quantities of the stress hormone ethylene. The compound 1-methylcyclopropene (1-MCP) inhibits the binding of ethylene to the ethylene receptor site, the ethylene binding protein (EBP). Treating infected fruit with 1-MCP prevented infection-induced degreening, such that fumigated fruit retained their green immature color compared to yellow non-fumigated controls. However, 1-MCP treatment significantly increased whole fruit ethylene production. In flavedo tissue of infected non-1-MCP treated fruit, 1-aminocyclopropane-1-carboxylate (ACC) synthase transcript accumulation, ACC synthase (ACS) enzyme activity, ACC and ethylene synthesis were all significantly higher +5 mm ahead of the lesion front than in uninfected non-1-MCP treated controls, but decreased significantly with increased sampling distance away from the lesion. 1-MCP treatment increased ethylene production in infected fruit at all three sampling distances compared to the non-fumigated samples. Even in the absence of infection, 1-MCP treatment resulted in increased ethylene synthesis. The results suggest that, in the presence of a pathogenic stress, blocking the EBPs prevented regulatory control of the ethylene biosynthetic pathway that resulted in an uninhibited expression of the ACS stress-associated genes, increased ACS activity and elevated ACC accumulation and ethylene production. Blocking of the EBPs with 1-MCP did not affect progression of the pathogen through the fruit. Published by Elsevier Science B.V.
Keywords:Ethylene; Ethylene binding protein; Grapefruit;Penicillium digitatum; 1-Methylcyclopropene
www.elsevier.com/locate/postharvbio
1. Introduction
Ethylene is associated with the regulation of several plant metabolic processes and is produced rapidly in response to pathogenic invasion (Boller, 1990). Indeed increased ethylene biosynthesis is a common feature of citrus infected with the com-mon postharvest pathogen Penicillium digitatum
(Achilea et al., 1985; Dutta and Biggs, 1991). Mention of a trademark, warranty, proprietary product,
or vendor does not constitute a guarantee by the U.S. Depart-ment of Agriculture and does not imply its approval to the exclusion of other products or vendors that may also be suitable.
* Corresponding author. Tel.: +1-814-8632074; fax: + 1-814-8637217.
E-mail address:[email protected] (E.D. Mullins)
The mechanisms by which plants perceive and transduce the ethylene signal are topics that have been researched intensively. Following the isola-tion of Arabidopsis ethylene insensitive mutants (Bleecker et al., 1988) and the cloning of two genes in particular involved in the pathway (ETR 1 gene, Chang et al., 1993; CTR 1 gene, Kieber et al., 1993), models of the chain of events that proceed ethylene binding have been proposed (Bleecker and Schaller, 1996; Pallin et al., 1996; Kieber, 1997; McGrath and Ecker, 1998). Subse-quent to ethylene binding with the ethylene bind-ing protein (EBP), a suspected heterotrimetric G-protein (Harpham et al., 1996), they suggest a negative regulation of the receptors His kinase activity which inactivates the activity of the CTR 1 protein kinase. It is predicted that this in turn influences through a MAP kinase cascade the downstream products, EIN 2, EIN 3, EIN 5 and ultimately ethylene sensitivity.
Several inhibitors of ethylene synthesis have assisted in the study of the pathway (Yu and Yang, 1980; Yoshii and Imaseki, 1982; Kende, 1989; Bouquin et al., 1997; Nakatsuka et al., 1997; Mullins et al., 1999). 1-MCP is a non-toxic compound that inhibits ethylene perception by irreversibly binding to and hence inactivating the EBP (Sisler et al., 1996). Shown to be an effective inhibitor of ethylene action in banana (Golding et al., 1998), Petunia hybrida (Serek, 1995) and
Gypsophila paniculata (Newman, 1998), this an-tagonist has been suggested as a possible alterna-tive for the commercially used silver thiosulfate (STS) in improving product longevity (Porat et al., 1995).
Wound-induced production of ethylene in cit-rus flavedo is strongly inhibited by treatment with ethylene (Riov and Yang 1982), a phenomenon referred to as ‘autoinhibition’. Investigations into the mechanisms by which ethylene modifies its own synthesis have focused on the action of ACS (Yoshii and Imaseki 1982; Yang and Hoffman 1984; Hyodo et al., 1985) and how autoinhibition, by acting at the level of ACS gene expression (Peck and Kende, 1998; Mullins et al., 1999), suppresses induction of the protein (Yang and Hoffman 1984; Hyodo et al., 1985) and subse-quently inhibits the accumulation of ACC (Riov
and Yang 1982; Hyodo et al., 1985). If however the first step of the pathway, binding of ethylene to the EBPs (Ecker, 1995), is interceded with by treatingP.digitatum-infected fruit with 1-MCP, it is unclear what the outcome would be with re-spect to the evolution of stress ethylene and the progression of disease through the host tissue. The aim of this research was to provide some answers to this question.
2. Materials and methods
2.1. Fruit source, inoculation and disease assessment
Grapefruit (Citrus paradisi) were collected from a local grove on a regular basis between October and December, washed under hot water to re-move residual crop protectants, and air dried prior to treatment.
On day 0, 20 min prior to fumigation, fruit were infected by piercing the flavedo to a depth of 2 mm and inoculating with a 5 ml spore suspen-sion (1×106
spores ml−1
water) of P. digitatum
(+INF). Controls were inoculated with sterile water (−INF) and all fruit were then incubated in the presence or absence of 1-MCP at 25°C for 4 days (Section 2.2). Disease progression in each fruit was assayed by measuring the average lesion diameter on day 2, 3 and 4. Two lesion diameters (each intersecting the point of inoculation and perpendicular to each other) were recorded per fruit.
2.2. Fruit fumigation
to stand in the dark for 24 h, 95% RH. Fumiga-tion was then repeated (day 2) as described for a further 16 h, after which the fruit were left to stand (day 3) for 24 h, before analysis commenced (day 4). Control chambers (−MCP) contained 4 ml of buffering agent.
2.3. Fruit color measurements
Peel color (three readings per fruit) was recorded using a Minolta Chromameter (Model CR-300, Minolta Camera Corp., Osaka, Japan) on day 0, 2 and 4 of the 4-day incubation period (Section 2.2). The Commission Internationale de l’Eclairage a* and b* color index scale was em-ployed and expressed as a ratio of a*/b* as described by McDonald et al. (1997). An increas-ing ‘a/b’ ratio, from −0.55 to −0.10, indicated increased yellow color with a ratio of −0.10 being considered the equivalent of mature colored grapefruit.
2.4. Ethylene determination
Ethylene evolution from whole fruit was moni-tored (six fruit per treatment) by incubating indi-vidual fruit in 1.75-l jars. After 90 min at 25°C, a 1-ml sample was extracted from the headspace of the jar via a rubber septum and injected into a gas chromatograph (Hewlett Packard, Model 5880A) equipped with an alumina packed column and a flame ionization detector. Results are expressed in nmol ethylene kg−1 h−1.
To determine ethylene production from flavedo, discs excised from healthy tissue at increasing distances (+5, +30, +55 mm) in front of the lesion were sealed in 130-ml jars (seven disks per jar per treatment with seven fruit assayed per treatment) which contained a disc of Whatman No. 1 filter paper pre-soaked with 1 ml of water. After 90 min at 25°C, a 1-ml sample was extracted from the headspace of the jar via a rubber septum and injected into the gas chromatograph for anal-ysis. Control discs were excised at the +5 mm distance from fruit inoculated with sterile water. Results are given in nmol ethylene kg−1 h−1.
2.5. ACC determination
Flavedo discs (1.5 g fresh wt.) were macerated in liquid nitrogen and resuspended in 80% ethanol (2 ml g−1). Samples were extracted with shaking (150 rev. min−1) at 25°C, after which the mixture was spun at 5000×g for 15 min and the superna-tant collected on ice. This extraction was repeated twice, the supernatants pooled, and the volumes recorded. After reducing to near dryness at 50°C, extracts were brought to a volume of 3 ml with H2O and centrifuged at 28 500×g for 5 min. To remove extracted pigments, 200 ml of chloroform was added and the volume spun at 28 500×g for 5 min. The organic phase was decanted off and to precipitate any protein present, 200 ml of phenol/ chloroform/isoamyl alcohol (25:24:1) was added and the suspension spun at 28 500×gfor 10 min. ACC in the aqueous phase was assayed by the method of Lizada and Yang (1979) using 250 mM HgCl2 and a 1.5-ml sample for injection into the gas chromatograph. Each sample was analyzed in triplicate with an internal standard containing 4 mM ACC. All steps were performed at 4°C and five fruit per treatment were tested. Recovery of ACC was typically between 60 and 80% with ACC obtained, expressed as mmol kg−1
.
2.6. Assay of ACC synthase (ACS) acti6ity
incubation buffer. Protein content was determined on this extract according to Lowry et al. (1951) with BSA as standard. ACC synthase (EC 4.4.1.14) activity was determined in a reaction mixture containing 3.67 ml desalted flavedo ex-tract, 375 ml incubation buffer and 450 ml of 500 mM S-adenosyl-L-methionine (AdoMet). After a 3-h incubation at 30°C, protein was eliminated from the mixture by adding 3.75 ml of phenol/ chloroform/isoamyl alcohol (25:24:1) followed by vortexing and then centrifuging for 10 min at 28 500×g. The aqueous phase was decanted and clarified by centrifuging at 28 500×g for 10 min; ACC formed was assayed by the method of Lizada and Yang (1979) using 50 mM HgCl2 and a 1.5-ml sample of headspace gas for injection into the GC. Each sample was analyzed in tripli-cate and contained one internal standard spiked with 4mM ACC. All steps were performed at 4°C and each treatment was assayed three times. En-zyme activity was expressed as mmol of ACC formed kg−1 protein h−1.
2.7. RNA extraction
Total RNA was extracted from 1 g flavedo disks using the guanidine – phenol – chlorofrom method described by Strommer et al. (1993) with slight modification. Tissue was ground to a pow-der unpow-der liquid N2, resuspended in RNA extrac-tion buffer (4 M guanidium isothiocyanate, 25 mM sodium citrate, 0.5% sarcosyl, 10 ml b-mer-captoethanol ml−1 extraction solution). The aqueous phase was extracted a second time with phenol – chloroform and RNA precipitated with an equal volume of isopropanol at −20°C. RNA was pelleted by centrifugation at 10 000×gfor 10 min. The RNA pellet was redissolved in 500 ml water, mixed with an equal volume of 4 M LiCl, and allowed to precipitate at 0°C for 1 h. The precipitated RNA was pelleted by centrifugation at 16 000×g for 10 min, washed 2× with 70% ethanol, air dried and dissolved in water.
2.8. Probe labeling
The ACS gene sequence, cloned and described by Mullins et al. (1999), was labeled with
digoxi-genin using the PCR digoxidigoxi-genin (DIG) probe synthesis kit (Boehringer Mannheim, Boehringer, Germany) following the manufacturer’s instruc-tions.
2.9. Northern blot analysis
Total RNA (10 mg) was fractionated in 1.5% agarose gel containing 2% formaldehyde. Follow-ing electrophoresis, the gels were equilibrated in 1× NaPO4– EDTA, pH 7 prior to transfer to nylon membrane. RNA was transferred by capil-lary in 1× NaPO4– EDTA, pH 7 onto positively charged nylon membrane (Boehringer Mannheim) and following transfer, RNA was UV-crosslinked to the membrane. Membranes were prehybridized in DIG Easy Hyb (Boehringer Mannheim) for 4 h at 50°C. DIG-labeled probe was denatured by boiling for 10 min, syringe filtered (0.45 mM) and then added to the hybridization solution to give a final concentration of 25 ng ml−1. Hybridization was carried out overnight at 50°C. Following hybridization, the membranes were washed twice in 2× SSC+0.1% SDS at room temperature for 15 min and then twice in 0.1× SSC+0.1% SDS at 55°C for 15 min. Probe, hybridized to the target, was detected using the DIG chemilumines-cent detection system (Boehringer Mannheim) fol-lowing the manufacturer’s instructions. All Northern blot experiments were completed in duplicate.
3. Results
3.1. Peel color changes
any ethylene, their degreening seemed to be a diverse event, that could possibly be as a result of the two successive 16-h confinement periods. In all treatments, degreening was most prominent between the second and fourth day of the incuba-tion period (Fig. 1).
3.2. Disease progression
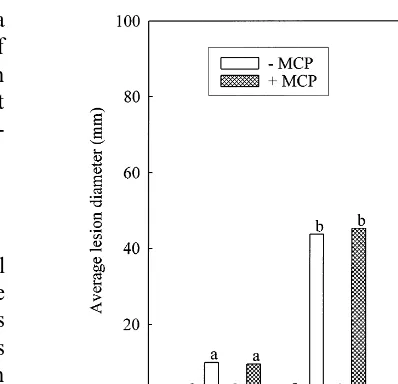
Analogous with the changes recorded in peel color was the progress ofP.digitatumthrough the host tissue. After 2 days of incubation there was no difference in lesion diameter among treatments (Fig. 2). However, after an additional 24 h, lesion diameters on the infected samples (with or with-out 1-MCP treatment) had increased fourfold. During the next 24 h, lesion diameters had in-creased eightfold. 1-MCP fumigation of grapefruit infected with P. digitatum did not induce any significant change in the susceptibility of the fruit to the disease (Fig. 2), or in its progression over the 4-day incubation period.
Fig. 2. Progression ofP.digitatuminfection in 1-MCP treated (+MCP) grapefruit. Fruit were uninoculated (−) or inocu-lated (+) on day 0 and showed initial symptoms of infection by day 2. Lesion diameter was recorded on day 2, 3, 4 of the 4-day incubation period, during which the fruit were fumi-gated twice (day 0 and day 2) and incubated at 25°C. Data are presented as the mean lesion diameter of 20 fruit per replicate (three replicates per treatment, four treatments per time pe-riod). Columns sharing the same letter within each time period are not significantly different (PB0.05) by the Tukey Test.
Fig. 1. Influence of 1-MCP on development of peel color inP. digitatum-infected grapefruit. Average color values of four treatments: −MCP +INF (), −MCP −INF ( ), +
MCP +INF () and +MCP −INF (). Over the 4-day incubation period, fruit were fumigated twice and the a*/b* ratio was determined on day 0, 2, and 4. Values are the mean9S.E. of three replicates (10 fruit per replicate, three replicates per treatment).
3.3. Ethylene and ACC biosynthesis
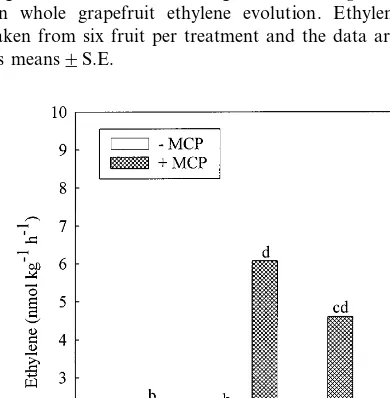
Evolution of ethylene from whole grapefruit was substantially increased following exposure to 1-MCP. Fumigation of grapefruit with 1-MCP increased ethylene production from 0.0834 to 1.823 nmol kg−1 h−1 (Fig. 3). This response was compounded when the fruit were infected withP.
digitatum. Infected fruit treated with 1-MCP pro-duced 6.276 compared to 1.06 nmol kg−1h−1for the non-fumigated infected fruit.
latter two distances and the corresponding −MCP−INF controls.
For all sampling points (+5, +30, +55 mm) of infected fruit, there was a difference (PB0.05)
Fig. 5. Accumulation of ACC in flavedo tissue excised from 1-MCP fumigated grapefruit (+MCP) at increasing distances in front of a P. digitatumlesion. Data are presented as the mean of five fruit per treatment. Columns sharing the same letter are not significantly different (PB0.05) by the Tukey Test.
Fig. 3. Effects of 1-MCP fumigation andP.digitatuminfection on whole grapefruit ethylene evolution. Ethylene readings taken from six fruit per treatment and the data are presented
as means9S.E. between the +MCP and corresponding −MCP
samples (Fig. 4). While ethylene production by
both the −MCP+INF and +MCP+INF
treated fruit declined with increased distance from the lesion, the reduction was more pronounced within the −MCP+INF samples than within the +MCP+INF samples.
Similarly, ACC, the immediate precursor of ethylene, accumulated more in +MCP+INF treated fruit than in −MCP+INF fruit at all three distances from the infection front (Fig. 5). ACC production was highest for both treatments at +5 mm but declined with increased distance from the lesion.
3.4. ACC synthase acti6ity and gene expression
In −MCP fruit,P.digitatuminfection induced an increase (PB0.05) in ACS activity at 5 mm in front of the lesion compared to the uninfected sample (Fig. 6). At the 30- and 55-mm sampling points, infection did not significantly influence enzyme activity in −MCP samples. 1-MCP fumi-gation of infected fruit amplified ACS activity Fig. 4. Ethylene evolution from flavedo discs excised from
significantly (PB0.05) at all three distances ahead of the lesion compared to the corresponding −MCP samples.
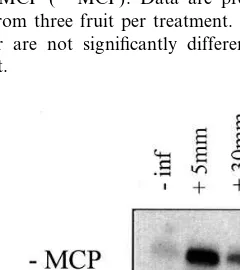
In the absence of 1-MCP, accumulation of ACS mRNA mirrored ACS activity (Figs. 6 and 7). The greatest abundance of ACS mRNA was ob-served at +5 mm. ACS mRNA decreased sub-stantially at +30 mm and further still at +55 mm. As with all of the previous experiments, the consequences of exposure of the tissue to 1-MCP was clear. Transcript accumulation, with the ex-ception of +5 mm where it was possible that maximum transcript accumulation had already been achieved, was increased substantially in both uninfected and infected samples in contrast to the −MCP treated samples. Furthermore, combining the data from Figs. 6 and 7 suggested the exis-tence of post-transcriptional control of ACS in flavedo tissue, particularly when the stress of P.
digitatum infection was absent.
4. Discussion
Fumigation of grapefruit with 1-MCP signifi-cantly enhances ethylene production. This has also been reported with banana (Golding et al., 1998). In the presence of fungal infection, this phenomenon is magnified further. P. digitatum is a serious postharvest pathogen of grapefruit which can completely rot the host within 3 – 4 days. A characteristic symptom of infection is an over-production of ethylene by the host (Achilea et al., 1985; Figs. 2 and 3), that results in the degreening of the peel with progression of the disease if the fruit is at an immature stage (Fig. 1). Following 1-MCP treatment however, fumigated fruit retained their green color (Fig. 1) in spite of synthesizing large quantities of ethylene (Figs. 3 and 4).
It is important that the signaling components in a given cascade are present at all times so that the cell is competent to respond to external stimuli. Because of this requirement, it may be advanta-geous for certain signaling pathways to possess a feedback mechanism to regulate the abundance of key components in the cascade. This would act to either enhance or desensitize the response of cells subjected to the constant presence of the same stimulus (Yohiharu et al., 1998). Sisler et al. (1996) have indicated that 1-MCP invariably Fig. 6. ACS activity in flavedo tissue, excised from uninfected
orP. digitatum-infected grapefruit treated with (+MCP) or without 1-MCP (−MCP). Data are presented as the mean collected from three fruit per treatment. Columns sharing the same letter are not significantly different (PB0.05) by the Tukey Test.
binds to the physiological ethylene receptor and it is hypothesized here that 1-MCP, while competi-tively inhibiting the ethylene binding protein (Sisler and Serek, 1997), represses the pathways signaling mechanism and effectively shuts down the system of feedback that modifies ethylene biosynthesis. Such events would thereafter lead to an uncontrolled increase in ethylene synthesis.
Ethylene production can be initiated by a vari-ety of developmental and environmental factors (Yang and Hoffman, 1984; Morgan and Drew, 1997). Upon experiencing stress, a cell typically synthesizes ethylene. In the absence of 1-MCP, these molecules are received by the EBPs, which may not necessarily be plasma membrane bound (Ecker, 1995). Ethylene binding then results in inactivation of the MAP kinase cascade, regulated by CTR 1, which subsequently allows for activa-tion of the signaling cascade (McGrath and Ecker, 1998). This ultimately alters gene expres-sion (Kieber, 1997) and coordinates ethylene syn-thesis while the ‘stress’ persists. However, in the presence of 1-MCP ethylene cannot bind. Conse-quently, no signal is received to control ethylene metabolism and the tissues continue to produce large amounts of ethylene as they fail to perceive the quantities already being synthesized.
To investigate this, ethylene biosynthesis was examined in flavedo tissues excised from grape-fruit that were treated in the presence or absence of 1-MCP and/or P.digitatum infection. In addi-tion, the analysis was completed at varying dis-tances ahead of the infected tissue so as to provide a comprehensive picture of the fruits re-sponse. In the absence of 1-MCP, P. digitatum
infection resulted in substantially higher ACS gene expression 5 mm ahead of the lesion front (Fig. 7). Correspondingly, this resulted in a sig-nificant increase in ACS activity (Fig. 6), ACC accumulation (Fig. 5) and hence ethylene produc-tion (Fig. 4). As the sampling distance increased ahead of the lesion front, synthesis of the compo-nents of the ethylene biosynthetic pathway de-creased sharply such that beyond 30 mm, there was no significant difference between these sam-ples and the non-infected tissues. The introduc-tion of 1-MCP however, amplified the tissue response at all sampling points such that at 55
mm (180° from the point of inoculation), there was a significant difference between the produc-tion of ACS activity, ACC accumulaproduc-tion and ethylene at this distance and at that of the unin-fected tissue. Even in the absence of infection, enzyme activity and product formation increased in discs from 1-MCP treated fruit.
While the treatment of whole fruit with 1-MCP could possibly be significant in terms of disease control, experiments showed that fumigation of fruit with 1-MCP did not impede progress of the pathogen or alter host susceptibility (Fig. 2). This appears to contradict recent results with ethylene-insensitive tobacco mutants (Knoester et al., 1998), but it is not believed that ethylene plays a major role in host-resistance (Bent et al., 1992). Instead, it is thought that ethylene could partici-pate as an ancillary component during host de-fense, possibly affecting expression of other more pathotoxic compounds or proteins (Boller, 1990; Beffa and Meins, 1996). Lund et al. (1998) indi-cated that ethylene perception was not important for regulation of tomato defenses against patho-gen infection during the early stage of the suscep-tible response. These data agree with this and imply that in grapefruit, ethylene perception was not a major factor in host defense at any stage in the infection process.
Northern blot analysis revealed that 1-MCP had a pronounced effect on ACS gene expression (Fig. 7). Maximum transcript accumulation was recorded for 1-MCP treated tissues, irrespective of
P. digitatuminfection. This was to be expected as it has been confirmed (Mullins et al., 1999) that ethylene exhibits an inhibition on ACS expression and that 1-MCP blocks ethylene perception (Sisler et al., 1996).
While our gene probe only detected a single band on the Northern blot, it is not suggested that the probe recognized only one transcript. Indeed, it is likely that more than one ACS associ-ated gene exists in citrus as has been reported in mungbean (Botella et al., 1992), tomato (Yip et al., 1992) and etiolated pea seedlings (Peck and Kende, 1998) where a multigene family coordi-nates ACS expression.
as a possible alternative to STS in extending the shelf-life of certain produce and flowers (Nell, 1992) and it has been suggested that the com-pound could be capable of controlling all ethylene responses in plants (Sisler and Serek, 1997). While 1-MCP does not confer any benefit to grapefruit in reducing P. digitatum infection it could be employed as an effective tool to study the interac-tion between ethylene and other defense-associ-ated responses.
References
Achilea, O., Chalutz, E., Fuchs, Y., Rot, I., 1985. Ethylene biosynthesis and related physiological changes inPenicil -lium digitatum-infected grapefruit (Citrus paradisi). Phys-iol. Plant Pathol. 26, 125 – 134.
Beffa, R., Meins, F., 1996. Pathogenesis-related functions of plant b-1,3-glucanases investigated by antisense transfor-mation — A review. Gene 179, 97 – 103.
Bent, A.J., Innes, R.W., Ecker, J.R., Staskawicz, B.J., 1992. Disease development in ethylene-insensitive Arabidopsis thaliana infected with virulent and avirulentPseudomonas andXanthomonaspathogens. Mol. Plant Microbe Interact. 5, 372 – 378.
Bleecker, A.B., Schaller, G.E., 1996. The mechanism of ethylene perception. Plant Physiol. 111, 653 – 660. Bleecker, A.B., Estelle, M.A., Somerville, C., Kende, H., 1988.
Insensitivity to ethylene conferred by a dominant mutation inArabidopsis thaliana. Science 241, 1086 – 1088.
Boller, T., 1990. Ethylene and plant-pathogen interactions. In: Flores, H.E., Arteca, R.N., Shannon, J.C. (Eds.), Polyamines and Ethylene: Biochemistry, Physiology and Interactions. American Society of Plant Physiologists, Rockville, MD, pp. 138 – 145.
Botella, J.R., Arteca, J.M., Schlagnhaufer, C.D., Arteca, R.N., Phillips, A.T., 1992. Identification and characterization of a full-length cDNA encoding for an auxin-induced 1-aminocyclopropane-1-carboxylate synthase from etiolated mung bean hypocotyl segments and expression of its mRNA in response to indole-3-acetic acid. Plant Mol. Biol. 20, 425 – 436.
Bouquin, T.B., Lasserre, E., Pradier, J., Pech, J., Balague, C., 1997. Wound and ethylene induction of the ACC oxidase melon gene CM-ACO1 occurs via two direct and indepen-dent transduction pathways. Plant Mol. Biol. 35, 1029 – 1035.
Chang, C., Kwok, S.F., Bleecker, A.B., Meyerowitz, E.M., 1993.Arabidopsisethylene-response gene ETR 1: Similarity of product to two-component regulators. Science 262, 539 – 544.
Dutta, S., Biggs, R.H., 1991. Regulation of ethylene biosyn-thesis in citrus leaves infected withXanthomonas campestris pv.citri. Phys. Plant. 82, 225 – 230.
Ecker, J.R., 1995. The ethylene signal transduction pathway in plants. Science 268, 667 – 675.
Golding, J.B., Shearer, D., Wyllie, S.G., McGlasson, W.B., 1998. Application of 1-1-MCP and propylene to identify ethylene-dependent ripening processes in mature banana fruit. Postharvest Biol. Technol. 14, 87 – 98.
Harpham, N.V.J., Berry, A.W., Holland, M.G., Moshkov, I.E., Smith, A.R., Hall, M.A., 1996. Ethylene binding sites in higher plants. Plant Growth Regul. 18, 71 – 77. Hyodo, H., Kuniaki, T., Yoshisaka, J., 1985. Induction of
1-aminocyclopropane-1-carboxylic acid synthase in wounded mesocarp tissue of winter squash fruit and the effects of ethylene. Plant Cell Physiol. 26, 161 – 167. Kende, H., 1989. Enzymes of ethylene biosynthesis. Plant
Physiol. 91, 1 – 4.
Kieber, J.J., 1997. The ethylene signal transduction pathway in Arabidopsis. J. Exp. Bot. 48, 211 – 218.
Kieber, J.J., Rothenberg, M., Roman, G., Feldmann, K.A., Ecker, J.R., 1993. CTR 1, a negative regulator of the ethylene response pathway inArabidopsis, encodes a mem-ber of the Raf family of protein kinases. Cell 72, 427 – 441. Knoester, M., Van Loon, L.C., Van den Heuvel, J., Hennig, J., Bol, J.F., 1998. Ethylene-insensitive tobacco lacks non-host resistance against soil-borne fungi. Proc. Natl. Acad. Sci. 95, 1933 – 1937.
Lizada, M.C., Yang, S.F., 1979. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal. Biochem. 100, 140 – 145.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J., 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265 – 275.
Lund, S.T., Stall, R.E., Klee, H.J., 1998. Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell 10, 371 – 382.
McDonald, R.E., Greany, P.D., Shaw, P.E., McCollum, T.G., 1997. Preharvest applications of gibberelic acid delay senescence of Florida grapefruit. J. Hort. Sci. 72, 461 – 468. McGrath, R.B., Ecker, J.R., 1998. Ethylene signaling in Ara-bidopsis thaliana: Events from the membrane to the nu-cleus. Plant Physiol. Biochem. 36, 103 – 113.
Morgan, P.W., Drew, M.C., 1997. Ethylene and plant re-sponses to stress. Phys. Plant. 100, 620 – 630.
Mullins, E.D., McCollum, T.G., McDonald, R.E., 1999. Ethylene: A regulator of stress induced ACC synthase activity in nonclimacteric fruit. Phys. Plant. 107, 1 – 7. Nakatsuka, A., Shiomi, S., Kubo, Y., Inaba, A., 1997.
Expres-sion and internal feedback regulation of ACC synthase and ACC oxidase genes in ripening tomato fruit. Plant Cell Physiol. 38, 1103 – 1110.
Nell, T.A., 1992. Taking silver safely out of the longevity picture. Grow. Talks 52, 35 – 38.
Newman, J.P., 1998. Evaluation of ethylene inhibitors for postharvest treatment of Gypsophila paniculata L. Hort. Technol. 8, 58 – 63.
Phosphorylation in Plants. Oxford Science, London, pp. 255 – 265.
Peck, S.C., Kende, H., 1998. Differential regulation of genes encoding 1-aminocyclopropane-1-carboxylate (ACC) syn-thase in etiolated pea seedlings: Effects of indole-3-acetic acid, wounding and ethylene. Plant Mol. Biol. 38, 977 – 982.
Porat, R., Shlomo, E., Serek, M., Sisler, E.C., Borochov, A., 1995. 1-Methylcyclopropene inhibits ethylene action in cut phlox flowers. Postharvest Biol. Technol. 6, 313 – 319. Riov, J., Yang, S.F., 1982. Effects of exogenous ethylene on
ethylene production in citrus leaf tissue. Plant Physiol. 70, 136 – 141.
Serek, M., 1995. Inhibition of ethylene-induced cellular senes-cence symptoms by 1-methylcyclopropene. Acta Hort. 405, 264 – 268.
Sisler, E.C., Serek, M., 1997. Inhibitors of ethylene responses in plants at the receptor level: Recent developments. Phys. Plant. 100, 577 – 582.
Sisler, E.C., Dupille, E., Serek, M., 1996. Effect of 1-methylcy-clopropene and methylenecyclopropane on ethylene
bind-ing and ethylene action on cut carnations. Plant Growth Regul. 18, 79 – 86.
Strommer, J., Gregerson, R., Vayda, M., 1993. Isolation and characterization of plant mRNA. In: Glick, B.R., Thomp-son, J.E. (Eds.), Methods in Plant Molecular Biology and Biotechnology. CRC Press, Boca Raton, FL, pp. 49 – 65. Yang, S.F., Hoffman, N.E., 1984. Ethylene biosynthesis and
its regulation in higher plants. Annu. Rev. Plant Physiol. 35, 115 – 189.
Yip, W., Moore, T., Yang, S.F., 1992. Differential accumula-tion of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions. Proc. Natl. Acad. Sci. 89, 2475 – 2479.
Yohiharu, Y.Y., Matsui, M., Deng, X., 1998. Positive feed-back in plant signaling pathways. Trends Pharmacol. Sci. 3, 374 – 375.
Yoshii, H., Imaseki, H., 1982. Regulation of auxin-induced ethylene biosynthesis: Repression of inductive formation of 1-aminocyclopropane-1-carboxylate synthase by ethylene. Plant Cell Physiol. 23, 639 – 649.
Yu, Y., Yang, S.F., 1980. Biosynthesis of wound ethylene. Plant Physiol. 66, 281 – 285.