THESIS
THE CAPABILITY OF
Cissus quadrangularis
EXTRACT
TO MAINTAIN HOMEOSTASIS BLOOD CALCIUM
LEVEL AS FRACTURE FEMUR THERAPY ON
OVARIECTOMIZED RAT (
Rattus norvegicus
)
By:
MUHAMMAD IMAM HAIKAL 061111237
FACULTY OF VETERINARY MEDICINE UNIVERSITAS AIRLANGGA
Has been assessed in Result Seminar Date: 13th August 2015
THE CAPABILITY OF Cissus quadrangularis EXTRACT TO MAINTAIN HOMEOSTASIS BLOOD CALCIUM LEVEL AS FRACTURE FEMUR
THERAPY ON OVARIECTOMIZED RAT (Rattus norvegicus)
Muhammad Imam Haikal
ABSTRACT
The aim of this research was to study the difference of homeostasis blood calcium level in fracture femur between osteoporosis bone comparing with normal bone and the capability of Cissus quadrangularis (CQ) extraction as fracture femur therapy to maintain homeostasis blood calcium level on ovariectomized rats (Rattus norvegicus). Animal used in this research were 24 female rats, that randomly separated into four groups T0(-) was control negative group without ovariectomy treatment, T0 (+) was control positive group, T1 was administered with 5.4 mg/kg BW raloxifene, T2 was administered with 750 mg/kg BW CQ extraction with six replication in each treatment. In tenth day ovariectomy surgery conducted, then in eighth week osteotomy surgery performed. The blood serum sample was examined in two time period, three rats examined in two weeks after osteotomy conducted and three rats in six weeks after osteotomy performed. Examination used three different rats for each treatment in second week and sixth week time period. The result showed there was no significant difference in the homeostasis blood calcium level between normal bone compared with osteoporosis bone (p>0.05). Cissus quadrangularis
plant extract proved have the capability to maintain blood calcium homeostasis with no significant difference result (p>0.05) compared with raloxifen treatment group.
ACKNOWLEDGEMENTS
Bismillahirahmanirrahim
Countless thanks and highest gratitude to Allah SWT the gracious and merciful, my creator. The One and Only, for its grace and wisdom so the author could finish this thesis which titled The Capability of Cissus quadrangularis Extract to Maintain Homeostasis Blood Calcium Level as Fracture Femur Therapy on Ovariectomized Rat (Rattus norvegicus). Shalawat and salam always dedicated to Prophet Muhammad S.A.W who lead us to the right path and bring us from the darkness to the lightness.
In this occasion I would like to give my gratitude and thank to Prof. Dr. H. Fasich, Apt. as the Rector of Universitas Airlangga for accepting my Bachelor Program study in Veterinary Medicine Major at Universitas Airlangga Faculty of Veterinary Medicine and also Prof. Hj. Romziah Sidik, DVM., Ph.D, as the Dean,
Dr. Anwar Ma’ruf, drh., M.Kes as the first Vice Dean, and Prof. Dr. Rr. Sri Pantja
Madyawati, drh., M.Si. as the head of academic division of Faculty of Veterinary Medicine, for giving me the chance to study in the Veterinary Medicine Major Bachelor Program.
I would like to thank to the examiner committee, Ira Sari Yudaniayanti, drh., MP. as a chairman of examiner and my research leader, Dr. Kadek Rachmawati, drh., M.Kes. as secretary of examiner and Hardany Primarizky, drh., MVM. as member of examiner for their time and willingness to examine and provide suggestion for completion of this thesis.
I would like to thank to Dr. Soeharsono, drh., M.Si. as my statistical consultant, for helping and giving me suggestion for completion of this thesis.
I would like to thank to all staff lecturer in Faculty of Veterinary Medicine Universitas Airlangga Surabaya for their precious knowledge during my study in this faculty.
I would like sincerely like to thank Mr. Bayu who had been help me by provided all the equipment and material that I needed during my research in Animal Hospital Faculty of Veterinary Medicine Universitas Airlangga.
I would like to say my special thank to my beloved parents, my father H. Nyuhadi Al Abdul Hadi and my mom Hj. Suminah for their inspiration, love, so much pray for me, advice, patience, passion, material and moral support and encouragement throughout my study and for my life since I was born and also my thanks to all my sisters Mba Umi, Mba Titin, Mba Yanny, Mba Fitri, Mba Sari for helping me in so many ways to support me and their pray until this thesis complete.
My gratitude to all my friends in IC 32+, Tika, Belga, Ogen, Bayu, Ari, Geby, Hadi, Tri, Pavi, Usi, Dea, Anisa, Dona and the other that I could not said one by one for the unforgettable moments and togetherness for four years during our study. My research partner Diga, Vidi, Kemala thanks for every help and our teamwork until this research finally complete. All my friends in ANDALAS, thanks for every experience and stories that we shared together.
I, as the author hope this thesis can be reference for further research. I know making this thesis is not easy. There are too many obstacles but I believe this is an advance process of my life to make me stronger than before and after through it all, now I realize that animals and human are created by God as the completion for each other to make a good mutualism life so we should respect what God has been given to us.
Surabaya, August 2015
TABLE OF CONTENTS
ABBREVIATIONS AND SYMBOLS ... xv
CHAPTER 1 INTRODUCTION ... 1
2.3.1 Pathogenesis of Osteoporosis ... 12
2.4 Estogen Deficiency ... 13
2.5 Bone Fracture Healing ... 14
2.5.1 Bone Fracture Healing on Osteoporosis ... 15
2.8 Cissus quadrangularis ... 19
2.8.1 Classification of Cissus quadrangularis ... 19
2.8.2 Habitat ... 20
3.1 Research Location and Timeline ... 24
3.2 Research Materials and Equipments ... 24
3.2.1 Research Materials ... 24
3.3.3 Osteoporotic Rat Model Procedure ... 26
3.3.3.1 Ovariectomy ... 26
3.3.3.2 Osteotomy ... 28
3.3.4 Sample Collecting and Examination Procedure ... 29
3.4 Experimental Design ... 29
3.5 Research Variables ... 30
3.5.1 Independent Variables ... 30
3.5.2 Dependent Variables ... 30
3.5.3 Control Variables ... 30
3.6 Data Analysis ... 30
3.7 Research Flowchart ... 31
CHAPTER 4 RESULT ... 32
CHAPTER 5 DISCUSSION ... 34
CHAPTER 6 CONCLUSION AND RECOMMENDATION ... 38
6.1 Conclusion ... 38
6.2 Recomendation ... 38
SUMMARY ... 39
REFERENCES ... 41
LIST OF TABLES
Table Pages
LIST OF FIGURES
Figure Pages
LIST OF APPENDIX
Appendix Pages
1. Examination data analysis result of blood calcium level ... 48
2. Blood calcium level laboratory test result ... 56
3. Documentation of research ovariectomy surgery ... 58
4. Documentation of research osteotomy surgery ... 60
5. Cissus quadrangularis extraction procedure ... 61
6. Blood calcium level test calcium (Arsenazo) reagent set ... 62
7. Dose calculation 1st and 2nd week ... 64
ABBREVIATIONS AND SYMBOLS
ANOVA : Analysis of Variance BMD : Bone Mineral Density
BW : BodyWeight
CMC-Na : Carboxymethyl Cellulose Sodium CQ : Cissus quadrangularis
et al : et alii
FSH : Follicle-Stimulating Hormone GI : Gastrointestinal
IL : Interleukin
LH : Luteinizing Hormone pH : Power of Hidrogen PTH : Parathyroid Hormone
SHBG : Sex Hormone-Binding Globulin SPSS : Statistic Product and Service Solution SERM : Selective Estrogen Receptor Modulators TGF : Transforming Growth Factor
1
CHAPTER 1 INTRODUCTION
1.1 Background
Osteoporosis is characterized by the loss of bone mass and strength that leads to fragility fractures, probably existed throughout human history but only recently became a major clinical problem as the increasing of human age (Raisz, 2005). It is a major growing health problem for elderly women associated with ovarian hormone deficiency following menopause and the most common cause of age related bone loss in women (Shirwaikar et al., 2010). The major cause of osteoporosis is a lack of certain hormones, particularly estrogen in women and androgen in men (Shirwaikar et al., 2003). One of the other factors that could be expected to be the cause of osteoporosis is due to the actions of female animals sterilization (ovariectomy) which is usually done on pets, both dogs and cats that will decrease estrogen level of the body. The effect of a decrease in estrogen level will increase bone resorption resulting in the occurrence of osteoporosis.
these conditions, calcium requirement is quite high because in addition to be used for the improvement of osteoporosis condition as well as to the process of fracture healing. It was feared would interfere with the process of blood calcium homeostasis and then caused metabolism disorders.
Calcium is an essential element in the human body and is necessary to many cell functions. It is a vital component of bone architecture and is required for deposition of bone mineral throughout life. Although the body stores more than 99% of its calcium in the bones and teeth, it is also found in the extracellular fluid or plasma. Bone resorption increased to restore plasma levels in time of the plasma level decreases. Sufficient intake of calcium is necessary to maintain this balance. Calcium is absorbed in the small intestines with the aid of vitamin D (Kasper et al., 2005).
According to Hardy et al (1993) the blood calcium level was significantly reduced immediately after fracture. The level of ionised calcium in the blood is important for calcification, it will be reduced immediately after fracture and increased thereafter, during development of callus to facilitate the process of fracture calcification. Both calcium and phosphorous are transported to blood from gastrointestinal cells. Mineral homeostasis requires for the transport of calcium, magnesium, and phosphate across their target cells in bone, intestine, and kidney.
Gastrointestinal (GI) tract, to regulate the bone calcium and phosphorus. The calcium and phosphorus components are derived from the blood and which is from nutritional sources (Kini and Nandeesh, 2012).
Several studies have been conducted on the influence of osteoporosis on fracture healing. Experimental studies of bone fracture healing in osteoporotic animal models have shown reduced callus mass and reduced strength when compared with healing of similar fractures in animals with normal bone mass (Walsh et al., 1997;Namkung et al., 2001). The number of osteoclasts in the fracture calluses of the osteoporotic bone was significantly higher. These findings indicate that osteoporosis influences the healing of fractures, which may contribute to delayed healing of the fracture (Islam et al., 2005).
Many synthetic agents such as estrogens in hormone replacement therapy, selective estrogen receptor modulators like raloxifen have been developed to treat osteoporosis. Enhancement of estrogen will raise the absorption of calcium in intestine. But each one of them is associated with side effects such as hypercalcemia, hypercalciurea, increase risk of endometrial and breast cancer, breast tenderness, menstruation, thromboembolic events, vaginal bleeding and hot flushes (Shirwaikar et al., 2003). Then, it would be most helpful to explore
naturally occurring substances especially of plant origin that could prevent bone loss and free from any adverse effects.
Cissus quadrangularis (CQ) has a role on estrogenic receptors of the bone in
uptake of the minerals calcium, sulpher and strontium (Mishra et al., 2010). Cissus quadrangularis has the effect of increasing the stimulation of all the cells
of mesenchyma origin, namely the fibroblasts, the chondroblasts and osteoblasts which gives the effect of an increasing of the fibroblastic phase (first week), collagen phase (second week) and osteochondroital phase (third and fourth weeks) (Mishra et al., 2010).
Based on the background above, it is necessary to do more research on the capability of Cissus quadrangularis extract to maintain homeostasis blood calcium level as fracture femur therapy on ovariectomized rat (Rattus norvegicus).
1.2 Statement of Problem
1. Is there a difference level of homeostasis of blood calcium levels in a fracture femur on ovariectomized rat (Rattus norvegicus) between osteoporosis bone and normal bone?
2. Does Cissus quadrangularis extract have the capability as fracture femur
therapy on ovariectomized rat (Rattus norvegicus) to maintain homeostasis blood calcium level?
1.3 Theoretical Base
clinical features of osteoporosis is a consequence of the increase occurrence of bone fractures (Kanis, 2010).
Giannoudis et al., (2007) showed experimental studies on the effect of osteoporosis on fracture healing have been carried out on ovariectomized rats. These studies have shown that ovariectomy significantly reduces bone mass and the mechanical strength of the bone. Fracture healing appears to be delayed with callus mineralization and biomechanical properties. Both estrogen and calcium deficiencies are important risk factors in the pathogenesis of osteoporosis. Ovariectomy plus calcium deficiency results in great decrease in bone volume (Mazzeo et al., 1988).
According to O’Loughlin and Morris (1998) research, ovariectomized rats were unable to achieve the same calcium balance or absorption as the normal rats. Ovariectomy reduced calcium balance due to increase faecal calcium excretion, a consequence of reduced intestinal calcium absorption. Trabecular bone thus would be the preferred source of the additional calcium required to maintain homeostasis when this cannot be accomplished by increase intestinal absorption or decreased urinary excretion (Khosla et al., 2010).
mechanisms to adapt its strength to the changing needs of growth and physical exercis. Old, damaged, and unneeded bone is removed by resorption, and new bone is deposited by formation. Diseases affecting either or both of these processes lead to disturbed calcium homeostasis (Peacock, 2010).
The extracts of CQ stem showed anti-inflammatory properties and were used in enhancing osteoblast proliferation, bone fracture healing, ossification of fetal bone and increasing the thickness of trabecular bone (Varoni et al., 2012). Extract of CQ contains a high percentage of calcium ions and phosphorus, both essential for bone growth. Calcium ions, phosphorous and phytoestrogens present in this plant extract used in the process of ossification and very useful in bone fracture healing process (Rao et al., 2007). The plant extract also facilitated extracellular matrix mineralization, which was more pronounced in the presence of osteogenic media and the study proved that CQ accelerates fracture healing and also causes early remodeling of fracture callus (Singh et al., 2013).
1.4 Aim of Research
1. To determine the difference level of homeostasis of blood calcium levels in fracture femur on ovariectomized rat (Rattus norvegicus) between osteoporosis bone and normal bone.
2. To determine the capability of Cissus quadrangularis extract as fracture
1.5 Outcome of Research
To provide knowledge about the homeostasis level of blood calcium level between normal bone compared with osteoporosis bone and the benefits of Cissus quadrangularis extract as alternative therapy medicine to maintain homeostasis blood calcium level in cases of osteoporotic fractures.
1.6 Hypothesis
1. There is no difference in the level of homeostasis of blood calcium levels in a
fracture femur on ovariectomized rat (Rattus norvegicus) between osteoporosis bone and normal bone.
8
CHAPTER 2 LITERATURE REVIEW
2.1 Bone
Bone or osseous tissue, is a connective tissue in which the matrix is hardened
by the deposition of calcium phosphate and other minerals. The hardening process
is called mineralization or calcification (Saladin, 2003). It is a living tissue that is
capable of remodeling and repairing itself when damaged. It is a specialized type
of connective tissue. Which provide the rigid supportive framework of the body
and forms a system of levers for locomotion (Aspinall and O'reilly, 2004). For
example the temporal bone or humerus, is an organ, as it is formed by several
types of tissues, including bone tissue, bone marrow, dense connective tissue, and
others (JR and Bacha, 2012).
Bone consist an extracellular matrix or ground subtance that contains the
protein osteonectin and collagen fibres (Aspinall and O'reilly, 2004). The matrix
of osseous tissue are organic and inorganic matter. The organic matter includes
collagen and various protein carbohydrate complexes such as
glycosaminoglycans, proteoglycans, and glycoproteins. The inorganic matter is
about 85% hydroxyapatite, a crystallized calcium phosphate salt, 10% calcium
carbonate, and lesser amounts of magnesium, sodium, potassium, fluoride, sulfate,
According to Brodsky and Persikov (2005) the functional component of the
bone includes growth factors and cytokines. The hardness and rigidity of bone is
due to the presence of mineral salt in the osteoid matrix, which is a crystalline
complex of calcium and phosphate (hydroxyapatite). Calcified bone contains
about 25% organic matrix, 5% water, and 70% inorganic mineral
(hydroxyapatite).
Bone remodeling is a lifelong process wherein old bone is removed from the
skeleton (a sub-process called bone resorption), and new bone is added (a
subprocess called ossification or bone formation). Remodeling involves
continuous removal of discrete packets of old bone, replacement of these packets
with newly synthesized proteinaceous matrix, and subsequent mineralization of
the matrix to form new bone (Fernández-Tresguerres-Hernández-Gil et al., 2006 ;
Fraher 1993). Normal bone remodeling cycle requires that the process of bone
resorption and bone formation take place in a coordinated fashion, which in turn
depends on the orderly development and activation of osteoclasts and osteoblasts,
respectively (Fraher, 1993).
The balance between bone resorption and bone deposition is determined by
the activities of these two principle cell types, namely, osteoclasts and osteoblasts.
Osteoblasts and osteoclasts, coupled together via paracrine cell signaling, are
referred to as bone remodeling units. The balance between bone resorption and
formation is influenced by such interrelated factors as genetic, mechanical,
2.2 Osteogenesis
Osteogenesis begins with osteoblast formation and secretion of type I
collagen, which makes up about 90% of the organic bone matrix, or the osteoid.
Once osteoblasts are active, they begin to produce large amounts of alkaline
phosphatase, a phosphate spliting enzyme that is release into the osteoid to initiate
the deposition of minerals (Potu et al., 2009).
Bone formation is complex but the three dimensional positioning of cells and
matrices is straightforward. As in any discussion of bone formation it is important
to keep in mind the distinction between bone as a tissue (bone cells and the
mineralized matrix) and bone as an organ (including several tissues such as bone,
cartilage, fibrous tissue, marrow and blood vessels). Normal bone develops using
only 2 mechanisms (Shapiro, 2008).
In intramembranous ossification, osteoblasts directly deposit bone matrix in
or beneath a membrane. The membrane is either mesenchymal, as in the
development of a flat bone of the skull, or periosteal, as in growth in diameter of a
long bone (JR and Bacha, 2012).
Endochondral bone formation describes the synthesis of bone on a
mineralized cartilage scaffold after epiphyseal and physeal cartilage have shaped
and elongated the developing organ. These mechanisms are also used in fracture
and osteotomy repair with the specific mechanism dependent on the mechanical
environment provided during repair. With intramembranous bone repair,
mesenchymal cells differentiate along a preosteoblast to osteoblast line while
followed by the endochondral sequence of bone formation. The terms
intramembranous and endochondral refer to the tissue being replaced, not to the
eventual bone synthesized which is the same in both mechanisms (Shapiro, 2008).
2.3 Osteoporosis
Osteoporosis is described by the World Health Organization as a progressive
systemic skeletal disease characterized by low bone mass and micro architectural
deterioration of bone tissue, with a consequent increase in bone fragility and
susceptibility to fracture (Kanis, 2010). Osteoporosis has often been defined as a
disease of decreased bone mass leading to fragile bones (Dambacher et al., 2004).
The clinical features of osteoporosis are a consequence of the fractures that
arise (Kanis, 2010). Diet, lifestyle, comorbidity (other diseases a person has), and
medications all seem to play a more important role than genetics in determining
osteoporosis risk (Neustadt and Pieczenik, 2012). Estrogen deficiency has direct
as well as indirect impacts on bone metabolism all of which promote
osteoclastogenesis (Sipos et al., 2008).
Estrogen deficiency accelerates the normal turnover of bone tissue, but the
net activity of bone resorbing cells (osteoclasts) is greater than that of bone
forming cells (osteoblasts). This gives rise to thinning of the cortices of bones,
thinning of trabecular bone and loss of trabecular elements. The architectural
changes weaken bone disproportionately compared to the loss of skeletal mass
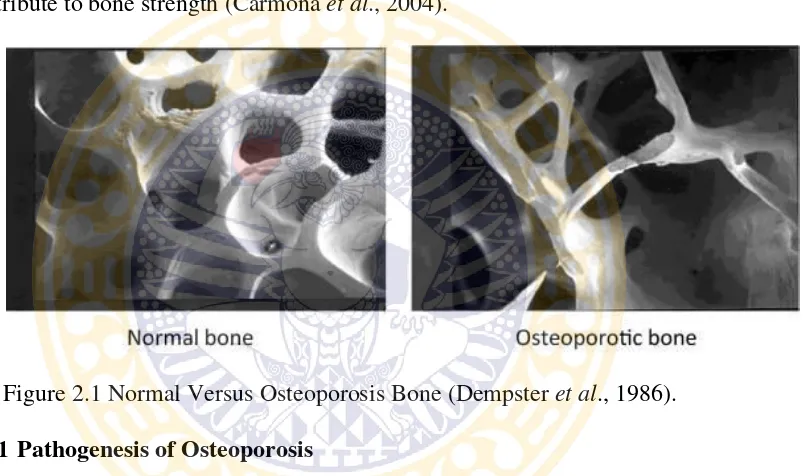
(Figure 2.1). The rate of loss of bone tissue is particularly rapid around the time of
throughout later life (age related or involutional bone loss) in men as well as
women (Kanis, 2010).
The structure of normal trabecular bone consists of well connected plates or
broad bands that provide great strength. In individuals with osteoporosis these
bands are disrupted and often become thin, weakened rods. Some of these rods are
no longer connected to another piece of bone, meaning that they no longer
contribute to bone strength (Carmona et al., 2004).
Figure 2.1 Normal Versus Osteoporosis Bone (Dempster et al., 1986).
2.3.1 Pathogenesis of Osteoporosis
It is well known the both estrogen and calcium deficiencies are important risk
factors in the pathogenesis of osteoporosis. Ovariectomy plus calcium deficiency
results in great decrease in bone volume, femoral weight, femoral ash weight and
cortical cross sectional area than did the calcium alone (Mazzeo et al., 1988).
According to Riggs and Melton (1986) statement, estrogen deficiency is
considered as the major determinant of bone loss in postmenopausal female. The
pathogenesis of postmenopausal osteoporosis is manifested by an increase in bone
osteoblasts. The excessive bone resorption by osteoclasts occurs without adequate
new bone formation by osteoblasts which lead to bone loss (Wronski et al., 1989).
Menopause result in elevated bone turnover, an imbalance between bone
formation and bone resorption and net bone loss. Ovariectomy in the rat results in
an increase in bone turnover rate and significant loss of cancellous bone such as
the proximal femur, vertebral bodies and the metaphysic of long bones (Omi and
Ezawa, 1995).
Normal bone turnover involves a balance between the processes of bone
resorption and bone formation in which osteoclasts remove (resorb) bone by
acidification and proteolytic digestion and osteoblast secrete osteoid (organic
matrix of bone) into resorption cavity. In postmenopausal female, the rate of bone
turnover increase dramatically and remains elevated for up to 40 years after
cessation of ovarian function, leading to continuous, progressive bone loss. The
basis for the increased bone turnover is thought to be due in part to a shortening of
the lifespan of osteoblasts and a prologation of the lifespan of osteoclasts (Lane,
2006).
2.4 Estrogen Deficiency
Estrogen acts on both osteoclasts and osteoblasts to inhibit bone breakdown at
all stages in life. Estrogen may also stimulate bone formation. The marked
decrease in estrogen at menopause is associated with rapid bone loss (Carmona et
A number of studies, both in vivo and in vitro, have implicated multiple
cytokines and other growth factors as being involved in estrogen effects on
osteoclast differentiation. Estrogen deficiency will lead to increased
osteoclastogenesis and continue to lose bone. This estrogen deficiency will
increase production of IL-6, IL-1 and or TNFα as potential mediators of osteoclast
differentiation. Estrogen also modulates transforming growth factor-β (TGF-ß)
production by osteoblasts, coupled with evidence that TGF-ß regulates osteoclast
differentiation (Oursler, 2003).
Estrogen also has extraskeletal effects in the form of decreased absorption of
calcium in the intestines, which leads to increased parathyroid hormone levels and
increased bone degradation in postmenopausal women (Meiyanti, 2010).
2.5 Bone Fracture Healing
The goal of fracture treatment is early ambulation and complete return of
function. A fracture is a complete or incomplete break in the continuity of bone or
cartilage and is accompanied by various degrees of injury to the surrounding soft
tissues, including blood supply, and by compromised function of the locomotor
system. The examiner handling the fracture must take into consideration the
patient’s local and overall conditions (Piermattei et al., 2006).
Fracture healing is a natural process that can reconstitute injured tissue and
recover its original function and form. It is a very complex process that involves
inflammatory cells, angioblasts, fibroblasts, chondroblasts and osteoblasts which
synthesize and release bioactive substances of extracellular matrix components.
Differentiation between primary or secondary fracture healing. Primary
healing occurs in cases of extreme stability and negligible gap size, involving a
direct attempt by the bone to form itself directly. Secondary healing occurs when
there is not enough stabilisation and gap size is moderate. In this case, healing
activates responses within the periosteum and external soft tissues that form an
external callus, which reduces the initial movement by increasing stiffness. Most
fractures are repaired by secondary healing, which does a more thorough job of
replacing old and damaged bone (Doblare et al., 2003).
Healing occurs in three distinct but overlapping stages: first the early
inflammatory stage, second the repair stage; and third the late remodeling stage
(Kalfas, 2001).
2.5.1 Bone Fracture Healing on Osteoporosis
During the healing process of the osteoporotic fracture, the bone resorption of
the trabecular bone formed from the intraperiosteal osteogenesis and
endochondral ossification was remarkably faster than that found in the normal
fracture healing, however, with slower and incomplete bone remodeling. After the
callus became mature, the woven bone remodeled in a way to adapt the local
mechanical requirement. This process was trigged by the osteoclast activation and
its together with the osteoblasts (Dai and Hao, 2007).
Osteoclastogenesis and osteoblastogenesis are the critical coordinated events
and bone formation and allow remodeling of the bone. The number of osteoclasts
in the fracture calluses of the osteoporotic bone was significantly higher at all
times than in the bone fracture without osteoporosis, indicating that osteoporosis
stimulates osteoclastogenesis during healing. Taken together with earlier
observations that loss of estrogen increased absorption of bone as a result of an
increase in osteoclastogenesis,these findings indicate that osteoporosis influences
the healing of fractures by upregulating the number of osteoclasts, which may
contribute to delayed healing of the fracture (Islam et al., 2005).
2.6 Raloxifene
Raloxifene is a nonsteroidal drug with partially agonistic estrogenic and
partially antiestrogenic properties. In postmenopausal women (whose endogenous
estrogen concentrations are very low) its estrogenic properties are utilized for the
prevention and treatment of osteoporosis.
In postmenopausal women with low endogenous estrogens, Raloxifene
decreases serum FSH without affecting serum LH or serum 17β-oestradiol,
whereas serum SHBG increases. These effects are interpreted as estrogenic
(Duschek and Netelenbos, 2004).
According to research Rey et al., (2009) in healthy postmenopausal women,
Raloxifene demonstrated that it significantly lowered bone turnover markers
during the 24 months that the study lasted (bone specific alkaline phosphatase by
15%, osteocalcin by 30%). Raloxifene is efficacious in the prevention and
osteoporosis, while at the same time, presenting a low incidence of side effects
and exhibiting a beneficial effect on breast tissue by decreasing the risk of breast
cancer. Raloxifene, the only SERM (Selective Estrogen Receptor Modulators) so
far approved for the prevention and treatment of osteoporosis, increases BMD
(Bone Mineral Density) less than do estrogens or bisphosphonates, but its
influence on fracture risk is similar (Tähtelä, 2004).
2.7 Calcium
Calcium as a nutrient is most commonly associated with the formation and
metabolism of bone. Over 99% of total body calcium is found as calcium
hydroxyapatite in bones and teeth, where it provides hard tissue with its strength.
Calcium in the circulatory system, extracellular fluid, muscle, and other tissues is
critical for mediating vascular contraction and vasodilatation, muscle function,
nerve transmission, intracellular signaling, and hormonal secretion. Bone tissue
serves as a reservoir for and source of calcium for these critical metabolic needs
through the process of bone remodeling. Excessive calcium resorption can
compromise the integrity and strength of the bone tissues (Ross et al., 2011).
The regulation of bone and bone mineral metabolism results from the
interactions of four hormones. It is parathyroid hormone (PTH), calcitonin (CT),
fibroblast growth factor 23 (FGF23) and vitamin D at bone, kidneys, and GI tract
to regulate calcium, magnesium, and phosphorus bone minerals.
Three calcium regulating hormones play an important role in producing
stimulates both resorption and formation of bone. Calcitriol is the hormone
derived from vitamin D, which stimulates the intestines to absorb enough calcium
and phosphorus and also affects bone directly. Calcitonin has a role for inhibits
bone breakdown and may protect against excessively high levels of calcium in the
blood (Carmona et al., 2004).
Bone calcium is controlled through the regulatory pathways of the
gastrointestinal (GI) tract and the kidneys, and this regulation is mediated in bone
by osteoblast as the bone forming cell and the osteoclast as the bone resorbing
cell. The GI tract can exhibit low calcium absorption, as in malabsorptive states,
or high calcium absorption as in vitamin D intoxication. The kidneys can
underexcrete calcium as occurs in some hypercalcemic disorders, overexcrete
calcium as in some patients with nephrolithiasis (Shaker and Deftos, 2000).
Xue and Fleet (2009) study showed calcium is absorbed by active transport
(transcellularly) and by passive diffusion (paracellularly) across the intestinal
mucosa. Active transport of calcium is dependent on the action of calcitriol and
the intestinal vitamin D receptor. Transcellular transport occurs primarily in the
duodenum where the intestinal vitamin D receptor is expressed in the highest
concentration. Passive diffusion or paracellular uptake involves the movement of
calcium between mucosal cells and more occur throughout the length of the
intestine during higher calcium intakes. However, the permeability of each
intestinal segment determines passive diffusion rates. The highest diffusion of
Calcium leaves the body mainly in urine and feces, but also in other body
tissues and fluids, such as sweat. Calcium excretion in the urine is a function of
the balance between the calcium load filtered by the kidneys and the efficiency of
reabsorption from the renal tubules (Hoenderop et al., 2000). In the intestine
calcium is excreted through the feces as unabsorbed intestinal calcium and is shed
in mucosal cells and secretions including saliva, gastric juices, pancreatic juice,
and bile (Ross et al., 2011).
2.8 Cissus quadrangularis
Cissus quadrangularis (Vitaceae), a rambling shrub, characterized by a thick
quadrangular fleshy stem, is an edible plant found in hotter parts of India, Sri
Lanka, Malaya, Java and West Africa (Udupa et al., 1970). Raj and Joseph (2011)
reasearch said CQ is commonly known as the “Bone Setter,” the plant is referred
to as “Hadjod” in Hindi because of its ability to join bones. A bioactive steroid is
believed to be the main constituent in CQ.
2.8.1 Classification of Cissus quadrangularis Kingdom : Plantae
Subkingdom : Tracheobionta
Super division : Spermatophyta
Division : Angiosperm
Class : Dicotyledoneae
Subclass : Rosidae
Genus : Cissus
Species : Cissus quadrangularis
(Shah, 2011).
2.8.2 Habitat
Found throughout the hotter parts of India alongside hedges, neighboring
countries like Pakistan, Bangladesh, Sri Lanka and Malaysia. It can be cultivated
in plains coastal areas, jungles and wastelands up to 500m elevation. Plant is
propagated using cuttings (Shah, 2011).
2.8.3 Morphology
Plant material occurs as pieces of varying lengths, tem quadrangular, 4
winged, internodes 4-15 cm long and 1-2 cm thick. The surface is smooth,
glabrous, buff colored with greenish tinge, angular portion reddish-brown, no
taste and odour. Leaves are simple 2.5-5 cm long, broadly ovate or reniform,
sometimes 3-7 lobed, denticulate, glabrous, cordate, rounded, truncate or cuneate
at the base, petioles 6-12 mm long, stipules small broadly ovate, obtuse (Figure
2.2). Flowers are in shortly peduncle cymes with spreading umbellate branches.
Calyx is cup shaped, truncate or very obscurely lobed. Petals are four, short, stout.
Berry is obovoid or globose, scarcely 6 mm, long apiculate, red when ripe,
Figure 2.2 Cissus quadrangularis (Raj and Joseph, 2011).
The whole plant including all parts such as stems, leaves, roots are
documented to possess medicinal properties in ethnobotanical surveys conducted
by ethnobotanists in traditional system of medicine (Shah, 2011).
2.8.4 Chemical Contain
Raj and Joseph (2011) observed studies on fracture healing suggest that the
steroid may act on estrogenic receptors of the bone. Also it has been observed that
CQ acts by stimulation of metabolism and increased uptake of the minerals
calcium, sulphur and strontium by the osteoblasts in fracture healing. The extract
of the plant exhibit cardiotonic and androgenic property. Phytochemical studies of
CQ found several phytochemical constituents such as ascorbic acid, carotene,
anabolic steroidal substances, calcium, β-sitosterol, δ-amyrin, δ-amyrone,
flavonoids, triterpenoids and various secondary metabolites. Pharmacological
studies showed the bone fracture healing property and antiosteoporotic effect of
2.9 Ovariectomy
Ovariectomy is a term used for ovarian removal. It could be unilateral
(partial) or bilateral (complete) when one ovary or both ovaries are removed
respectively (Alagwu and Nneli, 2005). The ovariectomized rat is an excellent
preclinical animal model that correctly emulates the important clinical feature of
the estrogen depleted human skeleton and the response of therapeutic agents. It is
sitespecific development of cancellous osteopenia or osteoporosis is one of the
most reproducible biologic responses in skeletal research (Jee and Yao, 2001).
2.10 Rattus norvegicus
The laboratory rat is the domesticated form of the species Rattus norvegicus,
the genus Rattus contains 66 species. Most species of Rattus are indigenous in
subtropical and tropical areas, R. norvegicus are cosmopolitan and can be found
on all continents (Koolhaas et al., 2010).
The maximum lifespan of wild rats kept in a semi natural enclosure is about
600 days for males and 700 days for females, whereas the median lifespan (50%
survival) is 300 days for males and 550 days for females (Calhoun, 1962).
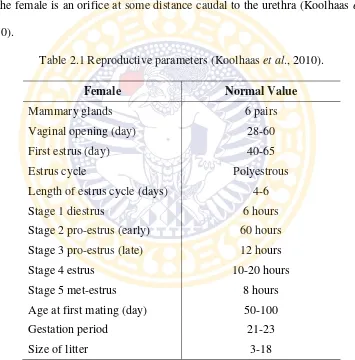
Both males and females are, on average, sexually mature at the age of 2-3
months. The estrus cycle is 4-5 days. At the time of estrus, receptivity occurs in
the second half of the dark period (Table 2.1) (Barbacka-Surowiak et al., 2003).
Wistar albino, this strain was developed at the Wistar Institute in 1906. The
It is an out bred or random bred strain and a large number of varieties exist
worldwide.
The sexes can readily be distinguished on the basis of the anogenital distance.
In males, the distance between the urethra and the anus is greater than in females.
Moreover, males can be distinguished by the wrinkled, sparsely haired scrotum at
the root of the tail. In a cool environment, the testes may be retracted. The vagina
of the female is an orifice at some distance caudal to the urethra (Koolhaas et al.,
2010).
Table 2.1 Reproductive parameters (Koolhaas et al., 2010).
Female Normal Value
Mammary glands 6 pairs
Vaginal opening (day) 28-60
First estrus (day) 40-65
Estrus cycle Polyestrous
Length of estrus cycle (days) 4-6
Stage 1 diestrus 6 hours
Stage 2 pro-estrus (early) 60 hours
Stage 3 pro-estrus (late) 12 hours
Stage 4 estrus 10-20 hours
Stage 5 met-estrus 8 hours
Age at first mating (day) 50-100
Gestation period 21-23
24
CHAPTER 3 MATERIALS AND METHODS
3.1 Research Location and Timeline
Research has been conducted in several different places, which were Animal
Hospital Universitas Airlangga, Assessment Service Unit of Pharmacy Faculty of
Universitas Airlangga, Balai Besar Laboratorium Kesehatan Daerah Surabaya.
This research has been held on March 2015 to July 2015.
3.2 Research Materials and Equipments 3.2.1 Research Materials
Stems and leaves of the CQ plant (collected from Purwodadi botanical
garden, Pasuruan), Raloxifene® (Elly Lily production), CMC Na 0.5%, ethanol
95%, alkohol 96%, alkohol 70%, aquadest, aquabidest, ketamine, xylazine,
iodium tincture, hypafix, antibiotics Enrofloxacin.
3.2.2 Laboratory Animal
Experimental animals used in this study were Twenty-four (24) females
Rattus Wistar strain that adapted in cage condition and feed. Aged two and half
months old with a weight range of 150-200 grams and in good health, which
marked with a shiny fur, eyes shining and active movement. which divided into
3.2.3 Research Equipments
Animal cage, disposable syringe 1 ml, disposable syringe 3 ml, digital scale,
microtome, oven microwave, ovariectomy surgical equipment set, osteotomy
surgical equipmennt set, intramedulary pin 1 mm – 1.2 mm, autoclave, gloves,
masker, centrifuge.
3.3 Research Procedure 3.3.1 Preparation of Extract
The fleshy stems and leaves were washed and cut into small pieces, air-dried
and crushed into a powder. The powder was extracted with 95% ethanol using a
Maceration extraction (Appendix 4).
3.3.2 Treatment
Twenty-four females Rattus norvegicus picked randomly and separated into
four group of treatment, as follows:
T0 (-) :group of non-ovariectomized rats and osteotomy with treatment
CMC Na 1.5 ml.
T0 (+) :group of ovariectomized rats and osteotomy with treatment CMC
Na 1.5 ml.
T1 :group of ovariectomized rats and osteotomy with administered
Raloxifene (5.4 mg/kg BW) (Potu et al., 2009).
T2 :group of ovariectomized rats and osteotomy with administered
First, all animal adapted to new cages for ten days. In day tenth ovariectomy
surgery performed for T0(+), T1, T2 rats. After ovariectomy surgery all animals
maintained for eight weeks to wait for the occurrence of osteoporosis caused by
estrogen deficiency. After eight weeks post ovariectomy all animals have been
done osteotomy surgery after radiological examination to make sure osteoporosis
have occurred and treatment in accordance with the administration continued for
six weeks ahead post osteotomy to provide healing process.
3.3.3 Osteoporotic Rat Model Procedure 3.3.3.1 Ovariectomy
The rats have been weighed and anesthetised using a combination of
ketamine (50 mg/kg BW) and xylazine (10 mg/kg BW) (Flecknell, 2009).
After the rats were anesthetised then pubis to arch costae extended to the left
and the right up to the mammary gland was shaved and sterilized with iodine
tincture. Rat was placed in the dorsal recumbency position. From the umbilicus to
the edge of the pelvis has been draped which aims to maintain sterility around the
area of surgery. The incision was made in the middle third of the caudal abdomen
through skin and subcutaneous tissue. The ventral rectus sheath was grasped,
tented it outward, and a stab incision was made into the abdominal cavity. The left
abdominal wall was elevated by grasping the linea with thumb forceps. Then
search the reproductive organs using spy hooks. Trace towards cranial of left horn
until the left ovary was found.
Two or three forceps were placed across the ovarian pedicle proximal to the
as a groove for the ligature, the middle clamp holds the pedicle for ligation, and
the distal clamp prevents backflow of blood after transection.
An absorbable suture material was chosen for ligatures blood vessel. Securely
the ligatures tied. A second circumferential ligature was placed proximal to the
first to control hemorrhage that may occur from puncturing a vessel. A mosquito
hemostat was placed on the suspensory ligament near the ovary. The ovarian
pedicle between the forcep and ovary transect. The ovary was examined to be
certain that it has been removed in its entirety. The forcep will be removed from
the ovarian pedicle and hemorrhage will be observed. The mesovarium would be
transect then proper ligament and the ovary was removed. The identical procedure
has been performed on the other side.
Post removal of the ovaries, abdominal cavity was irrigated using antibiotics
to prevent infection. Then, Peritoneum lining was closed with simple interrupted
sutures using chromic cat gut, skin was stitched with cross mattress pattern using
silk material. After the operation was completed the seam area would be swabbed
with iodine tinctures, and closed with hypafix.
This analogy was assumed to resemble a dog or cat events that have
ovariectomized and women who are experiencing menopause, which estrogen
deficiency occurs in these conditions. And according the study of Estai et al.,
(2011) had confirmed that six weeks of estrogen deficiency post-ovariectomy was
sufficient to cause significant bone loss in the rat model. Therefore, a period of
the control group to see the changes in bone density and osteoporosis
examinations in adult rats models to make sure osteoporosis have occurred.
3.3.3.2 Osteotomy
After the rats were confirmed osteoporosis on eighth week, then all the rats in
the group T0(-), T0(+), T1, T2 were performed osteotomy procedure in the
diaphysis of the femur with the installation intramedulary spin. Osteotomy
procedures using anesthesia with combination of ketamine (50 mg/kg BW) and
xylazine (4 mg/kg BW) (Flecknell, 2009).
Then rat was laid the lateral recumbency, the area to be operated given
antiseptic iodium tinture. To cover the area of operations sterile drape was
installed. Skin incision was done along the lateral line cranio of major trochanter
to the patella bone, the same thing was done with the subcutaneous tissue.
Retraction the skin and subcutaneous tissue, fascia lata was sliced along the edge
of the biceps femoris cranial muscular. After fascia sliced it would be seen the
muscular septum. Biceps femoris muscular was retracted to caudal and the vastus
lateralis muscular was pulled to cranial so it would be seen the surface of the
femur bone. Same manner was done for retraction adductor magnus muscular, it
was pulled to caudal and vastus interrmedius was fixed and drawn to cranial.
Bone stem was made separate from the surrounding muscular. Using bone saws,
bone at the center of the diaphysis of the femur was cut to transversely drop off.
Intramedular pin would be mounted in the following way. The pin to be
inserted first measured length was adjusted to the length of the femur bone based
femur through the end of the broken bone fragments at the distal and driven until
the pin cannot be moved again. The part of proximal bone fragment was taken and
the top end of the pin was pushed into the proximal bone fragment of the
medullary canal until the bone connected perfectly (fracture lines straight and the
bone ends do not overlap), then closed the operation wound.
For post-surgical care, the rats would be injected intra muscular with
Enrofloxacine 50 mg/kg body weight for two day. The CQ extract therapy, CMC
Na and Raloxifene were administered orally with feeding tube start from second
day until next six weeks.
3.3.4 Sample Collecting and Examination Procedure
All rats were taken from each group after two weeks and six weeks treatment.
Blood sample was taken from intracardiac with disposable syringe 3 ml as much
as 2 ml. Rat blood samples were put into a test tube was being tilted and wait for
10 minutes until serum come out. Serum has been centrifuged at 2500 rpm, at a
temperature of 3°C for 15 minutes. Serum going to inserted into the tube and
stored in the refrigerator at 4°C. Calcium serum level examination has been
performed in Balai Besar Laboratorium Kesehatan Daerah Surabaya.
3.4 Experimental Design
The experimental design for this research used Complete randomized design
factorial. with calculation formula (t-1) (n-1) ≥ 15. Then this research would be
3.5 Research Variables 3.5.1 Independent Variable
Independent variable used on this research are CQ extract and raloxifene drug
delivery after osteotomy.
3.5.2 Dependent Variable
Dependent variables in this research is blood calcium level on Rattus
norvegicus.
3.5.3 Control Variable
Control variable in this research are animal species, animal strain, type of
animal, animal age, animal sex, animal body weight, treatment of experimental
animals, animal cages, research place, research equipment, the selection of the
measuring instrument of research results, the materials are used in this research.
3.6 Data Analysis
Statistical analysis of data using SPSS 20.0 for Windows software (SPSS,
Chicago, IL, USA) The difference treatment groups were evaluated using
3.7 Research Flowchart
24 rats hospitalized for 10 days
2nd week post-osteotomy, 3 samples in each group were done blood calcium
level examination
In 10 days rats were divided according to the treatment
32
CHAPTER 4 RESEARCH RESULT
Blood sample collected from two different time period, and each time three
rats examined blood calcium level in Balai Besar Laboratorium Kesehatan Daerah
Surabaya. First sample examination was done 8th June 2015, the second
examination was done on 7th July 2015.
The result for this research the capability of Cissus quadrangularis extract as
fracture femur therapy on ovariectomized rat (Rattus norvegicus) to maintain
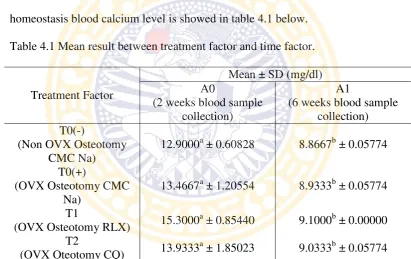
homeostasis blood calcium level is showed in table 4.1 below.
Table 4.1 Mean result between treatment factor and time factor.
Treatment Factor
Note: mean with different alphabetical superscript in the different column represent significant difference (p<0.05).
Data were analyzed using ANOVA (Appendix 1) showed the highest mean of
calcium in two weeks blood collection was T1 15.3000 ± 0.85440
(ovariectomized and osteotomy with administration Raloxifene), followed by T2
quadrangularis extract), then T0(+) 13.4667±1.20554 (ovariectomized and
osteotomy with administration CMC Na), and for the last was T0(-)
12.9000±0.60828 (non-ovariectomized osteotomy with administration CMC Na).
The highest mean result for sixth week blood collection was T1
9.1000±0.00000, followed by T2 9.0333±0.05774, then T0(+) 8.9333±0.05774,
and the lowest was T0(-) 8.8667±0.05774.
This research result indicates no significant difference between each
treatment in the same time factor (p>0.05) and no significant difference in
correlation of treatment and time factor (p>0.05). However, there were significant
34
CHAPTER 5 DISCUSSION
This research aimed to compare blood calcium level of femoral fracture healing process in ovariectomized rat (Rattus norvegicus) with Cissus quadrangularis extract therapy and to determine the difference level of
homeostasis of blood calcium levels in fracture femur on ovariectomized rat (Rattus norvegicus) between osteoporosis bone compared with normal bone.
In this studied there was not found significant difference between each treatment in the each blood collection time period, but between two weeks and six weeks time period blood sample collection there was found significant difference in blood calcium level result. Significant different was not found between each treatment in the same time period caused by the body will be always try to maintain the balance of calcium levels in the blood through a process of homeostasis.
Maintaining the level of circulating ionized calcium within a narrow physiological range is critical for the body to function normally and control of serum calcium levels is maintained through an endocrine system. A system of glands that secrete hormones is characterized by controlling factors and feedback mechanisms, includes a major role for vitamin D metabolites, calcitonin, and parathyroid hormone (PTH) (Sheweita and Khoshhal, 2007).
reduced serum calcium and acts peripherally on kidneys and bone and indirectly on the intestine to maintain serum calcium homeostasis (Raggatt and Partridge, 2010).
According to Shaker and Deftos (2000) The vitamin D metabolic system forms the basis of the calcium homeostatic mechanism in mammals. Total calcium concentration in serum is tightly regulated to remain between 8.5 and 10.5 mg/dL (2.12 and 2.62 mmol/L), and for normal blood calcium level in Rattus norvegicus wistar albino was 9,6-11 mg/dl (Kusumawati, 2004). Blood calcium
level for T0(-) and T0(+) in sixth week, were decreased below the normal value. This proves, control treatments have difficulties to restore Calcium levels to normal values compared with T1 and T2
If blood calcium serum level deviates slightly, the calcium sensing receptor of the parathyroid gland signals the secretion of PTH. Parathyroid hormone then stimulates the kidney to produce calcitriol, the hormonal form of vitamin D, as well as to activate bone resorption, which will increase extracellular calcium levels.
Calcitriol, through its receptor, also provides feedback relative to suppressing the production and release of PTH, commonly referred to as PTH suppression. Calcium is excreted mainly through the feces and urine to maintain normal calcium balance when exceeded (Ross et al., 2011).
Significant difference was found between each treatment on different time period mean result. The difference in two weeks healing process blood calcium relatively still remain in high level but after six weeks bone fracture healing process the blood calcium level significantly decreased to normal levels. During second weeks after fracture, bone fracture healing process still in inflammatory phase. In the inflammatory stage, a hematoma develops within the fracture site during the first few hours and days. Inflammatory cells (macrophages, monocytes, lymphocytes, and polymorphonuclear cells) and fibroblasts infiltrate the bone under prostaglandin mediation. This results in the formation of granulation tissue, ingrowth of vascular tissue, and migration of mesenchymal cells (Kalfas, 2001).
After the inflammatory phase was done, in the sixth week expected bone fracture healing proceed to reparative phase. The reparative phase occurs within the first few days, before the inflammatory phase subsides, and lasts for several weeks. The result of this phase will be the development of a reparative callus tissue in and around the fracture site. In this phase, pH gradually becomes neutral and then slightly alkaline, which is optimal for alkaline phosphatase activity and its role in the mineralization of the callus (Buckwalter et al., 1996).
endochondral ossification. Osteoblasts release membrane derived vesicles that contain calcium phosphate complexes into the matrix. Also carry neutral proteases and alkaline phosphatase enzymes in order to provide phosphate ions for precipitation with calcium.
As the mineralization process proceeds, the callus calcifies becoming more rigid and the fracture site is considered internally immobilize. This is followed by invasion of osteoblasts, which form primary spongiosa consisting of both cartilage and woven bone. Eventually the callus is composed of just woven bone, which connects the two fracture ends, and the remodeling process begins (Sfeir et al., 2005). The blood calcium level return to normal levels after it has been used in inflammatory phase, then the healing proceed to the remodeling phase with normal blood calcium level.
38
CHAPTER 6 CONCLUSION AND RECOMMENDATION
6.1 Conclusion
According to the research result can be summarized as follow:
1. There is no difference in the level of homeostasis of blood calcium level between normal bone compared with osteoporosis bone but there may be difference in healing rates of fracture of the femur.
2. Cissus quadrangularis extraction was proved have the capability as
fracture femur therapy to maintain homeostasis blood calcium level.
6.2 Recommendation
1. This research can be used for consideration for further research about determining the exact therapy dose of Cissus quadrangularis to gain its maximize effect.
2. Further radiological and bone qualities examination should be done to
provide more completed data and act as bone marker healing process. 3. Cissus quadrangularis can be used as alternative medicine with low
39 SUMMARY
Muhammad Imam Haikal. The research titled “The Capability of Cissus quadrangularis Extract to Maintain Homeostasis Blood Calcium Level as Fracture Femur Therapy on Ovariectomized Rat (Rattus norvegicus)” was under guidance of Prof. Dr. Fedik A Rantam, drh., as Supervisor and Dr. Rimayanti, drh., M.Kes. as Co-Supervisor.
The background of this research was begun from osteoporosis became major clinical problem for both humanity and animals. The major cause is due to estrogen deficiency from sterilization action (ovariectomy). Estrogen deficiency will lead to increase osteoclastogenesis and continue to lose bone that lead to fragility fractures. Estrogen deficiency also affects the decreased calcium absorption in the intestine.
Ovariectomy plus calcium deficiency are important risk factor in the pathogenesis of osteoporosis and results in great decrease in bone volume. One most important calcium functions is in skeletal mineralization. Thus, the homeostasis of blood calcium level should be maintain to support the fracture healing process. Extract of CQ contains a high percentage of calcium ions and phytoestrogen to used in ossification.
to determine the capability of CQ extract as fracture femur therapy on ovariectomized rat (Rattus norvegicus) to maintain homeostasis blood calcium level.
There were four treatment groups with following treatment design which each group was composed with 6 female rats (Rattus noervegicus) aged two and half month with weight rang 150-200 grams, good health and active condition. In ten days three group beside control negative group were done ovariectomy surgery then in eight week all rats would be done osteotomy surgery. In second days after osteotomy surgery, treatment administered for each group until six weeks. Blood calcium sample collected and examined in two different time period. Three rats examined in two weeks after osteotomy performed and the rest rats examined in six weeks after osteotomy conducted. The blood calcium examination was done in Balai Besar Laboratorium Kesehatan Daerah Surabaya.
Grouping of the experimental animal used Completely Randomized Design Factorial. The difference treatment groups were evaluated using ANOVA (Analysis of Variance) then followed by Duncan Located Test.
41
REFERENCES
Alagwu, E.A., and R.O. Nneli. 2005. Effect of Ovariectomy on The Levels of Plasma Sex Hormones in Albino Rats. Nigerian Journal of Physiological Sciences. 20(1-2):90-94.
Aspinall, V., and M. O'reilly. 2004. Introduction to Veterinary Anatomy and Physiology. Philadelphia. Usa: Elsevier Limited.
Brodsky, B., A.V. Persikov. 2005. Molecular Structure of The Collagen Triple Helix. Adv Protein Chem. 70:301–339.
Buckwalter, J.A., T. Einhorn, M.E. Bolander, R.L. Cruess. 1996. Healing of the Musculoskeletal Tissues. Rockwood and Green’s Fractures in Adults. Philadelphia. Lippincott-Raven Publishers.
Carmona, R.H., C. Beato, A. Lawrence, K. Moritsugu, A.S. Noonan. S.I. Katz. 2004. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD. U.S. Department of Health and Human Services, Office of the Surgeon General.
Dai, K.R., and Y.Q. Hao. 2007. Quality of Healing Compared Between Osteoporotic Fracture and Normal Traumatic Fracture. Advanced Bioimaging Technologies in Assessment of The Quality of Bone and Scaffold Materials. 531.
Dambacher, M.A., S. Schmitt, E. Schacht, M. Neff, R.L. Muller, L. Qin, Y.L. Zhao. 2004. Bone Structures In Vitro and In Vivo in Animals and in Men A View Into The Future. J Miner Stoffwechs. 11(3):13-19.
Dempster, D., E. Shane, W. Horbert, R. Lindsay. 1986. A Simple Method for Correlative Light and Scanning Electron Microscopy of Human Iliac Crest Bone Biopsies: Qualitative Observations in Normal and Osteoporotic Subjects. Journal of Bone and Mineral Research. 1(1):15-21.
Duschek, E.J., and L.J. Netelenbos. 2004. Effects of Raloxifene on Gonadotrophins, Sex Hormones, Bone Turnover and Lipids in Healthy Elderly Men. European Journal of Endocrinology. 150: 539–546.
Esa, T., S. Aprianti, M. Arif, and Haedjoeno. 2006. Nilai Rujukan Hematologi Pada Orang Dewasa Sehat Berdasarkan Sysmex Xt-1800i. Indonesian Journal of Clinical Pathology and Medical Laboratory. 127-130.
Estai, M.A., F. Suhaimi, I.N. Soelaiman, A.N. Shuid, S. Das. 2011. Bone Histomorphometric Study of Young Rats Following Oestrogen Deficiency. African Journal of Biotechnology. 12064-12070.
Fernández-Tresguerres-Hernández-GilI, Alobera-Gracia Ma, Del Canto-Pingarrón M. 2006. Physiological Bases of Bone Regeneration Ii. The Remodeling Process. Med Oral Patol Oral Cir Bucal. 11:E151–E157.
Flecknell, P. 2009. Laboratory Animal Anasthesia 3rd ed. Elsevier inc. London. Perspective. International Journal of The Injuried. 38(1):90-S99.
Goldhahn, J., D. Little, P. Mitchell, N. Fazzalari, I.R. Reid, P. Aspenberg, D. Marsh. 2003. Evidence for Anti-Osteoporosis Therapy in Acute Fracture Situations Recommendations of A Multidisciplinary Workshop of The International Society for Fracture Repair. Isfr Working Group Drugs and Fracture Repair .
Hoenderop, J., D. Muller, M. Suzuki, C.V. Os, R.J. Bindels. 2000. Epithelial Calcium Channel: Gate-Keeper of Active Calcium Reabsorption. Current Opinion in Nephrology and Hypertension.
Hardy, J.R., D. Conlan, S. Hay, P.J. Gregg. 1993. Serum Ionised Calcium and Its Relationship to Parathyroid Hormone After Tibial Fracture. Journal Bone Joint Surgery. 75-B:645-9.
Bone Morphogenetic Protein-2 and Tumour Necrosis Factor-Alpha. British Journal of Oral and Maxillofacial Surgery. 383-391.
Izzah, A., R.H. Ginardi, A. Saikhu. 2013. Pendekatan Algoritma Heuristik Dan Neural Network Untuk Screening Test Pada Urinalysis. Jurnal Cybermatika. 6.
Jee, W., and W. Yao. 2001. Overview: Animal Models of Osteopenia and Osteoporosis. Journal Musculoskel Neuron Interact. 1(3):193-207.
Jr, W.J., and L.M. Bacha,. 2012. Color Atlas of Veterinary Histology Third Edition. West Sussex, Uk: John Willey and Son Ltd.
Kalfas, I.H. 2001. Principles of Bone Healing. Neurosurg Focus 10. (4):1.
Kanis, J.A. 2010. Osteoporosis. Journal of Medical Sciences. 3(3):124-130.
Kasper, D.L., E. Braunwald, S. Hauser, D. Longo, J. Ameson, A.S. Fauci. 2005. Harrison's Principles of Internal Medicine 16th Edition Ii. New York: Mcgraw Medical Publishing Division.
Khosla, S., L.J. Iii, B.L. Riggs. 2010. The Unitary Model for Estrogen Deficiency and The Pathogenesis of Osteoporosis: Is A Revision Needed? Journal of Bone and Mineral Research. 26:441–451.
Kini, U., and B. Nandeesh. 2012. Physiology of Bone Formation, Remodeling, and Metabolism. in I. Fogelman, G. Gnanasegaran, and H. V. Wall, Radioncuclide and Hybrid Bone Imaging. 29-57.
Koolhaas, J.M., R. Hubrecht, J. Kirkwood. 2010. The Laboratory Rat, In The Ufaw Handbook on The Care and Management of Laboratory and Other Research Animals, Eighth Edition. Oxford,Uk: Wiley-Blackwell.
Krassas, G., and Papadopoulou. 2001. Oestrogen Action on Bone Cells. J Musculoskel Neuron Interact. 2(2):143-151.
Kusumawati, D. 2004. Bersahabat Dengan Hewan Coba. Gadjah Mada University Press. Yogyakarta. 8-10: 36: 50.
Lodish, H., A. Berk, S.L. Zipursky, P. Matsudaira, D. Baltimore, J. Damell. 2000. Molecular Cell Biology, 4th Edition. New York: W.H. Freeman.
Mazzeo, R.S., H.J. Donahue, S.M. Horvath. 1988. Endurance Training and Bone Loss in Calcium-Deficient and Ovariectomized Rats. Metabolism. 37(8): 741-744.
Meiyanti. 2010. Epidemiology of Osteoporosis in Postmenopausal Women Aged 47 to 60 Years. Grogol, Jakarta: Department of Pharmacy Medical Faculty, Trisakti University.
Mishra, G., S. Srivastava, B.P. Nagori. 2010. Pharmacological and Therapeutic Activity of Cissus quadrangularis: An Overview. International Journal of Pharmtech Research. 1298-1310.
Namkung, Matthai H., Appleyard R, Jansen J, Hao Lin J, Maas- Tricht S, Swain M, Mason Rs, Murrell Ga, Diwan Ad, Diamond T. 2001. Osteoporosis Influences The Early Period of Fracture Healing in A Rat Osteoporotic Model. 28(1):80-86.
Neustadt, J., and S. Pieczenik. 2012. Osteoporosis: Beyond Bone Mineral Density. JandS Media, Inc.
O’loughlin, P.D. and H.A. Morris. 1998. Oestrogen Deficiency Impairs Intestinal
Calcium Absorption in The Rat. Journal of Physiology. 313-322.
Omi, N., and I. Ezawa. 1995. The Effect of Ovariectomy on Bone Metabolism in Rats. Bone. 17:163-168.
Oursler, M. 2003. Direct and Indirect Effects of Estrogen on Osteoclasts. J Musculoskel Neuron Interact. 3(4):363-366.
Peacock, M. 2010. Calcium Metabolism in Health and Disease. Clinical Journal of The American Society of Nephrology.
Piermattei, Donald., G. Flo, C. Decamp. 2006. Brinker, Piermattei, and Flos’s Handbook of Small Animal Orthopedics and Fracture Repair. Fourth Edition. Saunders Elsevier. USA. 25.