DOI: 10.1542/peds.2007-0054
2008;121;766-776
Pediatrics
Carol Westall, Hilary Whyte and on behalf of the Postdischarge Feeding Study Group
Jefferies, Douglas M. Campbell, Elizabeth Asztalos, Mark Feldman, Joanne Rovet,
Deborah L. O'Connor, Sobia Khan, Karen Weishuhn, Jennifer Vaughan, Ann
With Extra Energy and Nutrients After Hospital Discharge
Growth and Nutrient Intakes of Human Milk Fed Preterm Infants Provided
http://www.pediatrics.org/cgi/content/full/121/4/766
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
rights reserved. Print ISSN: 0031-4005. Online ISSN: 1098-4275.
ARTICLE
Growth and Nutrient Intakes of Human Milk–Fed
Preterm Infants Provided With Extra Energy and
Nutrients After Hospital Discharge
Deborah L. O’Connor, PhD, RDa,b,c, Sobia Khan, MSc, RDa,b,c, Karen Weishuhn, RDa,b,c, Jennifer Vaughan, RN, IBCLCa,c, Ann Jefferies, MDd,e, Douglas M. Campbell, MDd,f, Elizabeth Asztalos, MDd,g, Mark Feldman, MDd,h, Joanne Rovet, PhDd, Carol Westall, PhDi, Hilary Whyte, MDd,j, on behalf of the Postdischarge Feeding Study Group
aPhysiology and Experimental Medicine Program,bDepartment of Clinical Dietetics, andjDepartment of Neonatology, Hospital for Sick Children, Toronto, Ontario,
Canada; Departments ofcNutritional Sciences,dPediatrics, andiOphthalmology and Vision Sciences, University of Toronto, Toronto, Ontario, Canada;eDepartment of
Pediatrics, Mount Sinai Hospital, Toronto, Ontario, Canada;fDepartment of Pediatrics, St Michael’s Hospital, Toronto, Ontario, Canada;gDepartment of Newborn and
Developmental Pediatrics, Sunnybrook Hospital, Toronto, Ontario, Canada;hDepartment of Paediatrics, St Joseph’s Health Centre, Toronto, Ontario, Canada
The authors have indicated they have no financial relationships relevant to this article to disclose.
What’s Known on This Subject
Although more could be done to improve the nutritional status of low birth weight (LBW) infants during their initial hospitalization, it is apparent that correction of acquired nutrient-deficits for many very LBW infants is difficult to accomplish before discharge.
What This Study Adds
Adding a multi-nutrient fortifier to⬃half of the milk provided to predominantly human milk-fed infants for 12 weeks after discharge may be an effective strategy in addressing early discharge nutrient-deficits without unduly influencing human milk feeding when lactation support is provided.
ABSTRACT
OBJECTIVES.The purpose of this pilot study was to determine whether mixing a multi-nutrient fortifier to approximately one half of the human milk fed each day for a finite period after discharge improves the nutrient intake and growth of predomi-nantly human milk–fed low birth weight infants. We also assessed the impact of this intervention on the exclusivity of human milk feeding.
METHODS.Human milk–fed (ⱖ80% feeding per day) low birth weight (750 –1800 g) infants (n⫽39) were randomly assigned at hospital discharge to either a control or an intervention group. Infants in the control group were discharged from the hospital on unfortified human milk. Nutrient enrichment of human milk in the intervention group was achieved by mixing approximately one half of the human milk provided each day with a powdered multinutrient human milk fortifier for 12 weeks after discharge. Milk with added nutrients was estimated to contain⬃80 kcal (336 kJ) and 2.2 g protein/100 mL plus other nutrients. Intensive lactation support was provided to both groups.
RESULTS.Infants in the intervention group were longer during the study period, and those bornⱕ1250 g had larger head circumferences than infants in the control group. There was a trend toward infants in the intervention group to be heavier at the end of the intervention compared with those in the control group. Mean protein, zinc, calcium, phosphorus, and vitamins A and D intakes were higher in the intervention group.
CONCLUSIONS.Results from this study suggest that adding a multinutrient fortifier to approximately one half of the milk provided to predominantly human milk–fed infants for 12 weeks after hospital discharge may be an effective strategy in addressing early
discharge nutrient deficits and poor growth without unduly influencing human milk feeding when intensive lactation support is provided.
I
N GENERAL, THEearlier an infant is born before his or her expected term delivery date, the greater is their riskfor morbidity and malnutrition.1,2The reasons for undernutrition are widely known to clinicians and include
that these low birth weight (LBW) infants are born with compromised nutrient reserves. Preterm infants often acquire nutrient deficits because initiation of parenteral nutrition and intralipids is frequently delayed; paren-teral glucose and lipid solutions are poorly tolerated; and feedings are frequently withheld for clinical proce-dures, sepsis, or suspicion of necrotizing enterocolitis.3Furthermore, comorbidities such as chronic lung disease limit the volume of feeding and/or route that nutrients are supplied, further complicating the provision of adequate nutrition.
www.pediatrics.org/cgi/doi/10.1542/ peds.2007-0054
doi:10.1542/peds.2007-0054
This trial has been registered at www. clinicaltrials.gov (identifier NCT00413985).
Key Words
low birth weight, premature infant, breastfeeding, human milk, growth
Abbreviations
LBW—low birth weight GTA— greater Toronto area CA— corrected age SGA—small for gestational age SAE—serious adverse event VLBW—very low birth weight
Although more could be done to improve the nutri-tional status and growth of LBW infants during their initial hospitalization, it is becoming increasingly appar-ent that correction of acquired nutriappar-ent deficits is diffi-cult to accomplish before discharge. In fact, many LBW infants leave the hospital with poorer nutritional status than when they started their postnatal life.3–8For exam-ple, using weight-for-age centiles (⬍10th centile) as a crude indicator of nutritional status, Lemons et al6 dem-onstrated that whereas 22% of the 4500 VLBW infants who were born at 1 of the US Neonatal Network sites were at risk for undernutrition at birth,⬃96% were so around the time of discharge. Furthermore, human milk–fed infants often accrue the greatest nutritional deficits by discharge.9A plethora of review articles and position statements from authoritative bodies under-score the concern about the nutritional status of human milk–fed LBW infants after hospital discharge.2–4,10–13In fact, the European Society for Paediatric Gastroenterol-ogy, Hepatology and Nutrition Committee on Nutrition recently recommended that human milk–fed preterm infants who are discharged from the hospital with sub-normal weight for postconceptional age be routinely supplemented to provide an adequate nutrient supply.11 Despite the aforementioned concerns, recommendation, and the widely known advantages of human milk over formula feeding,14no randomized, controlled trials have been conducted to ascertain whether multinutrient for-tification of human milk after hospital would be benefi-cial. The objectives of this pilot study, then, were to determine whether mixing a multinutrient fortifier to approximately one half of mother’s milk for 12 weeks after discharge would improve the nutrient intakes and growth of LBW (750 –1800 g) infants. Because few data specific to the LBW infant exist suggesting what impact this intervention might have on human milk feeding, we also assessed the duration and exclusivity of human milk use.
METHODS
Study Population
Primarily and exclusively human milk–fed infants and their mothers were enrolled between April 2004 and November 2005 from NICUs located in the greater To-ronto area (GTA). These nurseries included Mount Sinai Hospital, Sunnybrook Hospital, The Credit Valley Hospi-tal, St Joseph’s Health Center, St Michael’s HospiHospi-tal, Scarborough Hospitals (Grace and General Divisions), Scarborough Centenary (Rouge Valley Health System), and Toronto East General Hospital. Singleton or twin
infants (born ⬍33 weeks’ gestational age and between
750 and 1800 g) who received ⱖ80% of their total
feedings as human milk (fortified or unfortified) 3 days before hospital discharge were eligible to participate. Families agreed to feed their infants primarily human milk after hospital discharge and to supplement a pre-determined volume of the human milk fed with a pow-dered multinutrient fortifier for 12 weeks if so randomly assigned. Infants with serious congenital or chromo-somal anomalies that could affect growth were not
eli-gible to participate. Other exclusion criteria were grade 3 or 4 periventricular or intraventricular hemorrhage, oral steroids within 14 days of randomization, severe as-phyxia (hypoxia or ischemia characterized by an Apgar score of⬍5 at 5 minutes), and known maternal alcohol or drug abuse. Families were also ineligible when their principal residence was outside the GTA or when the mother was unable to communicate verbally in English. Last, infants were excluded from participation when any single feeding at hospital discharge needed to be nutrient enriched or concentrated to⬎24 kcal/fl oz (3800 kJ/L)
or when ⬎50% of the daily feeds required nutrient
enrichment.
Experimental Design
The Human Ethics Committees at the Hospital for Sick Children and each of the aforementioned recruiting hos-pitals approved the study, which was conducted accord-ing to the policies and procedures of each institution and the Canadian Tri-council policy statement on ethical conduct of research involving human subjects.15A com-puter-generated randomization schedule stratified for gender and birth weight group (ⱕ1250 g,⬎1250 g) was prepared by 1 of the authors (Dr O’Connor), who was not involved in enrolling study participants. A separate randomization schedule was prepared for twins. For avoidance of biasing in-hospital feeding practices or which individuals were approached about the study, randomization assignments were placed in individually sealed envelopes that were opened the day before hos-pital discharge (study day 1).
Infants who were randomly assigned to the control group were discharged from the hospital on unfortified human milk (from breast or expressed), as is routine clinical practice in the GTA. For infants who were ran-domly assigned to the intervention group, our goal was to supply roughly half the volume of human milk as nutrient-enriched feedings after hospital discharge. Nu-trient enrichment of human milk provided to infants in the intervention group was achieved by having
caregiv-ers mix a predetermined volume (150 mL ⫻ infant
weight [kg]/2) of human milk (fresh or thawed) with a powdered multinutrient human milk fortifier (4 single-use packets [0.9 g each] per 100 mL of human milk; Table 1). Remaining feedings were to be provided as unfortified milk (from the breast or expressed). Using the milk intake data collected from term-corrected age (CA) breastfed preterm infants published by Wauben et al,16we estimated that infants in this study would
con-sume⬃150 mL/kg per day around the time of hospital
discharge. The exact volume (150 mL ⫻infant weight
[3100 kJ/L] and 18 g/L protein).2Families could choose when during the day they wished to provide the nutri-ent-enriched feedings and whether they would use a bottle or supplemental nursing system.* Most families elected to use bottles. As is routine clinical practice in the GTA, infants in the control group were provided with vitamin drops consisting of vitamins A (1500 IU), D (400 IU), and C (30 mg) after discharge. For reduction of the possibility of inappropriately high intakes of fat soluble vitamins, infants who were randomly assigned to the intervention group were not provided with vitamins A and C and only 200 IU of vitamin D (ie, half the man-ufacturer recommended dosage of D-Vi-Sol [Mead Johnson Nutritionals, Ottawa, Ontario, Canada]). A daily iron supplement (15 mg/day) was prescribed for infants in both feeding groups.
An algorithm was created before study initiation to assess whether nutrient enrichment should be initiated for an infant in the control group who demonstrated poor growth. In general, when an infant’s growth, as
assessed by weight gain over ⬎7-day period or 2
con-secutive home visits, dropped by 2 percentile curves on the Infant Health and Development growth charts,17,18a feeding intervention was initiated. The same was true when an infant demonstrated clinical signs (eg, low tone, lethargy) of poor intake and growth. It was at the
discretion of the infant’s pediatrician how nutrient en-richment was to be accomplished, but, in general, pow-dered postdischarge formula (eg, Similac Neosure) was added to human milk. The study coordinator, a certified lactation consultant, offered lactation support to moth-ers in both feeding groups. Mothmoth-ers in both study groups were provided free of charge the vitamin/iron drops, feeding devices, and use of a double breast-expression electric breast pump (Purely Yours Breast Pump; Ameda, Mississauga, Ontario, Canada). Mothers in the interven-tion group were also provided the powdered human milk fortifier free of charge.
Demographic Data, In-hospital Tolerance, and Morbidity
Family characteristics of enrolled infants were obtained from a personal interview with 1 or both parents. This included mother’s gravidity and parity as well as paren-tal age, height, ethnicity, and years of education. Each infant’s weight and gestational age at birth, size at birth (ie, small for gestational age [SGA]or appropriate for gestational age), and in-hospital course were obtained from medical charts. The last included the number of days that parental nutrition was provided, days to reach full enteral feeds (100 kcal/kg per day [420 kJ/kg per day]), and days the infant received nothing by mouth after reaching full enteral feeds. Morbidity outcomes such as the number of infants with a confirmed case of necrotizing enterocolitis (more than Bell stage II), sys-temic infection (positive blood culture), or chronic lung disease before study day 1 were also recorded. Chronic lung disease was defined as the need for supplemental oxygen beyond 1 month chronological age or 36 weeks’ postconception.
The number of serious adverse events (SAEs) or un-expected adverse events was determined from study day 1 until 12 weeks after hospital discharge. An SAE was defined as any event that occurred during the clinical trial that resulted in death or was life-threatening or disabling, required hospital admission, or required inter-vention to prevent permanent impairment.
Growth
The weight, length, and head circumference of infants was determined according to standardized procedures by the study coordinator at study day 1 and at 4 (⫾3 days), 8 (⫾3 days), and 12 (⫾3 days) weeks after hospital
discharge during home visits.19 Infants were weighed
twice in the nude using a precision scale (⫾2 g; Medela BabyWeigh; Medela, Mississauga, Ontario). Recumbent length and head circumference were measured twice to the nearest 0.1 cm with a lengthboard (Ellard Instru-mentation, Munroe, WA) and nonstretchable tape mea-sure (InserTape, Ross Canada, Abbott Laboratories, Montreal, Quebec, Canada), respectively.
Enteral Intake
Dietary intake diaries were mailed to parents 3 days before each home visit (4, 8, and 12 weeks after dis-charge). Information collected in these diaries included intake of human milk consumed at the breast,
unforti-*Usually made up of a plastic container serving as a reservoir for supplemental milk and a capillary tube extending from the reservoir and placed adjacent to the mother’s nipple.
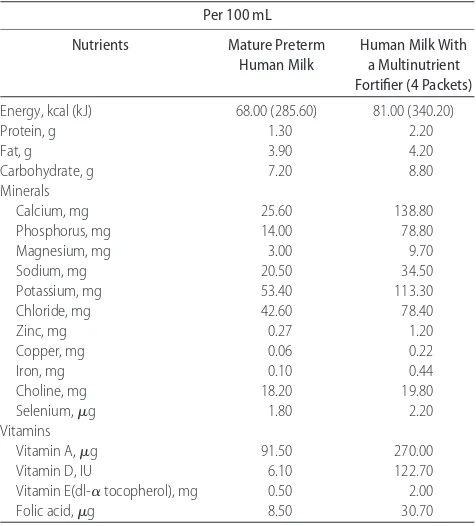
TABLE 1 Approximate Energy and Select Nutrient Composition of Mature Human Milk With and Without a Multinutrient Fortifier
Energy, kcal (kJ) 68.00 (285.60) 81.00 (340.20)
Protein, g 1.30 2.20
Selenium,g 1.80 2.20
Vitamins
Vitamin A,g 91.50 270.00
Vitamin D, IU 6.10 122.70
Vitamin E(dl-␣tocopherol), mg 0.50 2.00
Folic acid,g 8.50 30.70
Milk nutrient composition values were obtained from the literature.44 – 46Where possible, milk
fied expressed breast milk, nutrient-fortified human milk, formula, cow milk, and juice. Parents were taught to record their infant’s fluid intake regardless of source (eg, breast milk, expressed milk, formula, other) using a previously validated and standardized test-weighing procedure.20The test-weighing protocol required a care-giver to weigh infants before and after every feeding for 3 consecutive days before the 4-, 8-, and 12-weeks post-discharge home visit, using the Medela BabyWeigh scale, which was specifically designed for this purpose. The increase in weight after each feeding (in grams) provides an estimate of the amount of milk (in millili-ters) consumed. Parents were instructed not to change the infant or add or remove blankets or clothing be-tween the pre- and postfeeding weights. Use of vitamin and mineral drops was also recorded in the food diaries. Energy and select nutrient intakes (protein, calcium, phosphorus, zinc, iron, vitamin A, and vitamin D) were estimated using human milk composition values from the literature, manufacturer label claims for infant for-mulas and the powdered human milk fortifier, and the Canadian Nutrient File.21
To capture changes in human milk feeding frequency between 3-day food diaries, the study coordinator at the 4-, 8-, and 12-week postdischarge home visits asked mothers to estimate the number of times each day that her infant was fed since hospital discharge or the last visit and the number of these feeding that were provided as human milk. When the mother was no longer pro-viding human milk, she was asked to recall the date when human milk feeding was discontinued.
Statistical Analysis
We estimated a priori that 34 infants who completed the feeding intervention of this pilot study would allow us to detect a 1-SD difference in the mean weight of infants in the 2 feeding groups at 12 weeks after hospital discharge
with 80% power at an ␣ level of .05. All data were
analyzed using SAS 9.1 for Windows (SAS Institute, Cary, NC). All statistical tests were 2-tailed using an
␣-level of .05. Data were checked to ensure that they were normally distributed (PROC UNIVARIATE) and, as appropriate, transformed. Demographic data were ana-lyzed using ttests for continuous variables and2tests for categorical variables. Continuous outcome data
col-lected at ⬎1 time point were analyzed using mixed
repeated-measures analysis of variance, which accom-modates missing observations controlling for gender and birth weight strata (750 –1250 g or 1251–1800 g [PROC MIXED]). Growth rates from study day 1 to 4 weeks, study day 1 to 8 weeks, and study day 1 to 12 weeks were individually analyzed using analysis of variance (PROC GLM) controlling for gender and birth weight strata. Volume of human milk consumed at the breast and the intake of fortified human milk were not nor-mally distributed; hence, nonparametric pairwise com-parisons were completed for these 2 variables at each time point (Wilcoxon rank-sum test).
Given that infants who are SGA account for 25% of the preterm population in some nurseries, we elected to in-clude these infants in this study. However, preterm infants
who are SGA often exhibit a different growth pattern than preterm infants who are appropriate for gestational age, so all statistical analyses were rerun excluding infants who were SGA to ensure that their inclusion did not influence the study findings.22 In these latter statistical analyses, 1 infant who developed hydrocephalus during the study in the intervention group was also excluded.
RESULTS
Study Sample
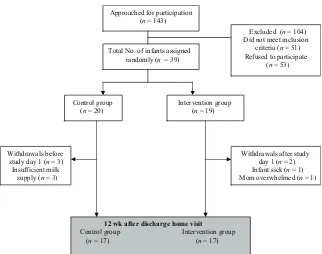
Families of 143 infants who were known to be primarily human milk fed were provided an information letter about the study. Of these infants, 51 did not meet the eligibility criteria and 53 refused to participate. Of the infants who met the eligibility criteria, 29 were excluded because their primary residence was outside the GTA and/or their mother decided to formula feed after hos-pital discharge. Mothers of 22 infants did not want to use bottles, a breast pump, or a human milk fortifier. Of the 53 eligible infants, the main reasons for refusing to par-ticipate were (1) the study was too much for them or they were busy with other children at home, (2) it was too far to travel to clinic visits planned for after the feeding intervention phase, and (3) they were already participating in other research studies (Fig 1).
For the remaining 39 infants, 20 were randomly as-signed to the control group and 19 to the intervention group. We had sufficient data on 34 infants at 12 weeks after discharge to conduct an intention-to-treat analysis. There were 5 withdrawals in the study: 3 in the control group before study day 1 because of insufficient milk supply and 2 in the intervention group after study day 1 but before the 4-week home visit because of a sick infant and an overwhelmed mother who chose to withdraw from the study.
Infant and Family Demographics
Most baseline infant and family demographics, including infant weight, length, and head circumference, at study day 1 did not differ statistically between the 2 feeding groups (Table 2). There was a trend toward older
gesta-tional age at birth (29.8 ⫾ 1.7 weeks) in the control
versus intervention group (28.9⫾1.2 weeks;P ⫽.06)
and fewer male infants in the control (11 of 20) versus
intervention group (14 of 19; P ⫽ .07). The potential
impact of these trends on outcomes was addressed by including the randomization strata (birth weight and gender) in all of the statistical analyses.
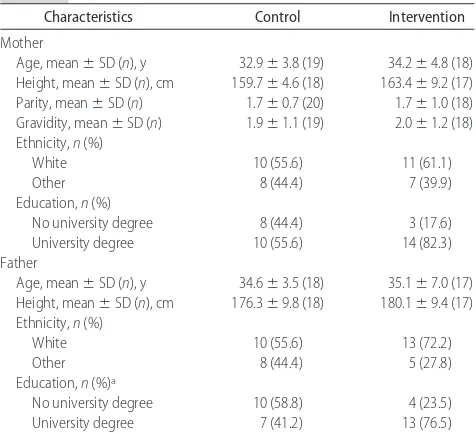
Infants and their families were primarily white, and parents were well educated (Table 3). Domperidone (Motilium; Loudwater, High Wycombe, United King-dom), a galactagogue prescribed as part of usual clinical practice in the GTA, was used by a significant number of
women in both feeding groups (control:n⫽12;
inter-vention:n⫽14) after hospital discharge.23
Growth
in the control group (Table 4). Among infants who were
ⱕ1250 g, those in the intervention group had a larger mean head circumference than those in the control
group (P⫽.0009). The adjusted mean head
circumfer-ences of infants who wereⱕ1250 g in the control group were 32.5⫾0.4, 34.7⫾0.4, 36.6⫾0.4, and 38.0⫾0.4 cm at study day 1 and at 4, 8, and 12 weeks after discharge, respectively. Likewise, the adjusted mean head circumferences of infants who wereⱕ1250 g in the intervention group were 34.0⫾0.3, 37.0⫾0.3, 39.0⫾
0.3, and 40.6⫾0.3 cm at study day 1 and at 4, 8, and 12
weeks after discharge, respectively. No difference in mean head circumference existed between feeding groups for infants born⬎1250 g.
The aforementioned statistical analyses included all infants as randomly assigned (intention-to-treat) includ-ing 2 infants in the control group who were fed human milk that contained powdered discharge formula to in-crease the nutrient concentration of the human milk fed to address slow growth. All statistical analyses controlled for gender and birth weight strata (750 –1250 g or 1251– 1800 g). Re-analysis of these growth data without the
TABLE 2 Neonatal Characteristics of the Study Population of Preterm Infants
Characteristics Control Intervention
Birth weight, mean⫾SD (n), g 1322⫾332 (20) 1253⫾242 (19) Gestational age at birth, mean⫾SD (n), wka 29.8⫾1.7 (20) 28.9⫾1.2 (19)
Male gender,n(%)b 11 (46.7) 14 (73.6)
Birth weight strata (ⱕ1250g),n(%) 12 (60.0) 8 (42.0) Weight at study day 1, mean⫾SD (n), g 2598⫾535 (17) 2785⫾465 (18) Postconceptional age at study day 1, mean⫾SD (n), wk 38.4⫾2.4 (17) 37.8⫾3.3 (18) Days on parenteral nutrition, mean⫾SD (n) 11⫾7 (20) 12⫾8 (19) Days to full enteral feeds (100 kcal/kg per day), mean⫾SD (n) 11⫾7 (20) 12⫾7 (19) Days feedings withheld, mean⫾SD (n) 0.6⫾1.6 (20) 0.1⫾0.2 (19)
Chronic lung disease,n(%) 5 (25.0) 8 (42.0)
Confirmed cases of NEC,n(%) 1 (5.0) 0 (0.0)
Confirmed cases of systemic infection,n(%) 1 (5.0) 1 (5.3) Size at birth,n(%)
SGA 2 (10.0) 1 (5.3)
Appropriate for gestational age 18 (90.0) 18 (94.7)
Multiple birth status,n(%)
Singleton 16 (80.0) 15 (78.9)
Twin 4 (20.0) 4 (21.1)
Differences between feeding groups for continuous variables were assessed by 2-sidedttests and for categorical variables using2analyses.
Mean⫾SD values presented are unadjusted. NEC indicates necrotizing enterocolitis.
aP⫽.06.
bP⫽.07.
Total No. of infants assigned
Control group (n = 20)
Intervention group (n = 19)
12 wk after discharge home visit Control group Intervention group (n = 17) (n = 17) Withdrawals before
study day 1 (n = 3) Insufficient milk
supply (n = 3)
Withdrawals after study day 1 (n = 2) Infant sick (n = 1) Mom overwhelmed (n = 1) Approached for participation
(n = 143)
Excluded (n = 104) Did not meet inclusion criteria (n = 51) Refused to participate
(n = 53) randomly (n = 39)
FIGURE 1
infants who were SGA (n⫽3) and a child who devel-oped hydrocephalus after randomization did not appre-ciably change the results for length and head circumfer-ence, but the trend for a difference in weight between the 2 feeding groups disappeared.
A statistically significant difference was found between
feeding groups among infants ⱕ1250 g in length gains
(cm/wk) between study day 1 and 12 weeks after discharge (P⫽.009). There was a trend toward infants in the
inter-vention group having more rapid head circumference gains versus the control group from study day 1 to 8 weeks after discharge (P⫽.09). This difference in head circumference gain was highly significant for infants bornⱕ1250 g up to 4 weeks after discharge (P ⬍ .0001). No differences in weight gains were observed between the feeding groups at any interval after discharge. When study day 1 anthropo-metrics (weight length or head circumference as appropri-ate) were added as a covariate to the statistical models, the results for length did not change, but a statistically signifi-cant difference in head circumference gain between feed-ing groups was observed from study day 1 to 12 weeks after discharge for infants bornⱕ1250 g (P⫽.03). There was a trend toward infants in the intervention group having greater weight gains to 4 weeks after discharge compared with infants in the control group (P⫽.05). These observed differences in length and head circumference gains early after hospital discharge are consistent with previous obser-vations that infants are at greatest nutritional risk at this time.9,24 Re-analysis of the aforementioned growth gains without the infants who were SGA and a child who devel-oped hydrocephalus after randomization did not apprecia-bly change the results, and, if anything, P values were strengthened.
Enteral Intake
Most infants consumed at least 1 human milk feeding per day at the 12-week postdischarge visit; only 1 infant in the control group and a set of twins in the interven-tion group did not. Likewise, the percentage of daily feedings provided as human milk at the 4-, 8-, and 12-week postdischarge home visits did not differ
be-tween the control (81 ⫾ 37%, 71 ⫾ 40%, and 71 ⫾
38%) and intervention (82⫾27%, 93⫾10%, and 88⫾
TABLE 3 Family Characteristicsa
Characteristics Control Intervention
White 10 (55.6) 11 (61.1)
Other 8 (44.4) 7 (39.9)
Education,n(%)
No university degree 8 (44.4) 3 (17.6) University degree 10 (55.6) 14 (82.3) Father
Age, mean⫾SD (n), y 34.6⫾3.5 (18) 35.1⫾7.0 (17) Height, mean⫾SD (n), cm 176.3⫾9.8 (18) 180.1⫾9.4 (17) Ethnicity,n(%)
White 10 (55.6) 13 (72.2)
Other 8 (44.4) 5 (27.8)
Education,n(%)a
No university degree 10 (58.8) 4 (23.5) University degree 7 (41.2) 13 (76.5)
Mean⫾SD values presented are unadjusted. Differences between feeding groups for contin-uous variables were assessed by 2-sidedttests and for categorical variables using2analyses. aP⫽.04.
TABLE 4 Weight, Length and Head Circumference Measurements from Study Day 1 to 12 Weeks After Hospital Discharge
Head circumference, cm ⬍.001 ⬍.0001 ⬍.001b
Study day 1 33.5⫾1.4 (17) 34.1⫾1.2 (17) 4 wk 35.9⫾1.3 (17) 37.1⫾1.0 (17) 8 wk 37.9⫾1.3 (17) 39.1⫾1.0 (17) 12 wk 39.3⫾1.5 (17) 40.5⫾1.0 (17)
Mean⫾SD values presented are unadjusted. Differences between feeding groups were assessed by repeated measures analysis of variance (ANOVA) controlling or gender and birth weight strata (ⱕ1250 g,⬎1250 g). NS indicates not significant.
aAn interaction between feeding group and time was found (P⫽.06). Intervention⬎control at 8 (P⫽.10) and 12 weeks (P⫽.07) postdischarge.
bInfants bornⱕ1250 g in the intervention group had larger heads than those in the control group during the study (P⫽.0009). Unadjusted
mean⫾SD head circumference; control (n⫽6): 32.4⫾1.2, 34.6⫾0.6, 36.5⫾0.7, and 37.9⫾1.4 cm; intervention (n⫽9): 34.1⫾1.2, 37.1⫾
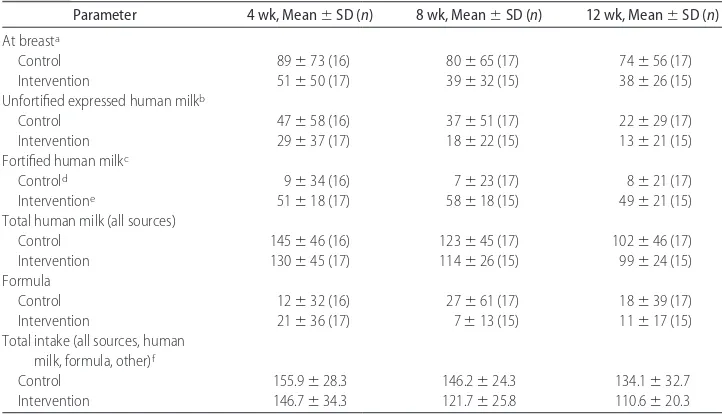
15%) groups. Furthermore, the total volume of human milk consumed (all sources) did not differ between feed-ing groups (Table 5). As planned, approximately half of the total human milk consumed each day in the inter-vention group had human milk fortifier added. None of the aforementioned enteral intake results differed when the infants who were SGA and the infant with hydro-cephalus were removed from the statistical analyses.
The mean volume of feedings (mL/kg per day) from
any source (human milk⫹formula⫹other) was greater
in the control than the intervention group at the 4-, 8-,
and 12-week home visits (P⫽ .02). Except for energy
and iron intake, mean nutrient intakes of infants in the intervention group were greater than that of infants in the control group (Table 6). Because the iron content of both human milk and the human milk fortifier used in this study was low, the primary source of iron came from iron drops. These results did not change appreciably when the statistical analyses were rerun without the infants who were SGA and the infant with hydroceph-alus; however, the difference in protein intake was no longer statistically significant (P ⫽ .06). The nutrient intakes of study infants were also compared with the recommended intakes for postdischarge feeding of pre-term infants published by the Canadian Paediatric Soci-ety.25Mean energy, protein, zinc, and iron intakes gen-erally were just at or below recommended levels for both feeding groups (Table 6). Mean vitamin D and calcium intakes generally exceeded the recommended levels in the intervention but not in the control group.
For confirmation that intakes of vitamin A and D re-mained within a safe intake range among infants who were consuming nutrient-enriched human milk, intakes were compared with adverse effect levels as published by the Institute of Medicine Dietary Reference Intakes for term-born infants.26,27This comparison demonstrated that
mean vitamin D intakes (diet ⫹ vitamin and mineral
drops) remained below the no observed adverse effect level of 1800 IU/day for infants 0 to 12 months.26 Likewise, mean vitamin A remained below the lowest observed ad-verse effect level of 6000g/day preformed vitamin A for infants who are younger than 1 year.27
Serious Adverse Events
Only 1 infant who was randomly assigned to the inter-vention group had an SAE, which consisted of 2 hospital admissions as a result of gastroesophageal disease. He was withdrawn soon after study day 1 at the request of his parents. This infant had not yet received the study human milk fortifier after hospital discharge.
DISCUSSION
Growth
Data from this pilot study suggest that LBW (⬍1800 g) infants who were fed human milk that contained extra nutrients for 12 weeks after hospital discharge were longer during the study period (P⫽.02), and those born
ⱕ1250 g had larger head circumferences than infants
who were sent home on human milk alone (P⫽.0009).
TABLE 5 Intake Volumes (mL/kg per D) by Feeding Type at 4, 8, and 12 Weeks After Hospital Discharge
Parameter 4 wk, Mean⫾SD (n) 8 wk, Mean⫾SD (n) 12 wk, Mean⫾SD (n) At breasta
Control 89⫾73 (16) 80⫾65 (17) 74⫾56 (17)
Intervention 51⫾50 (17) 39⫾32 (15) 38⫾26 (15)
Unfortified expressed human milkb
Control 47⫾58 (16) 37⫾51 (17) 22⫾29 (17)
Intervention 29⫾37 (17) 18⫾22 (15) 13⫾21 (15)
Fortified human milkc
Controld 9
⫾34 (16) 7⫾23 (17) 8⫾21 (17)
Interventione 51
⫾18 (17) 58⫾18 (15) 49⫾21 (15) Total human milk (all sources)
Control 145⫾46 (16) 123⫾45 (17) 102⫾46 (17)
Intervention 130⫾45 (17) 114⫾26 (15) 99⫾24 (15) Formula
Control 12⫾32 (16) 27⫾61 (17) 18⫾39 (17)
Intervention 21⫾36 (17) 7⫾13 (15) 11⫾17 (15)
Total intake (all sources, human milk, formula, other)f
Control 155.9⫾28.3 146.2⫾24.3 134.1⫾32.7
Intervention 146.7⫾34.3 121.7⫾25.8 110.6⫾20.3
Mean⫾SD values presented are unadjusted. Differences between feeding groups were assessed by repeated measures ANOVA controlling for gender and birth weight strata (ⱕ1250 g,⬎1250 g) except for milk consumed at the breast and fortified human milk, which were analyzed at each time point by a Wilcoxon rank-sum test. Unless otherwise indicated, no statistically significant differences were noted between feeding groups.
a4 weeks only:P⫽.06.
bControl⬎intervention,P⫽.03.
cIntervention
⬎control,P⬍.0001.
dPowdered postdischarge formula (eg, Neosure) was added to expressed human milk to a concentration of 24 kcal/oz (3300 kJ/L). eSimilac Human Milk Fortifier was added to expressed human milk to a concentration of 24 kcal/oz (3300 kJ/L).
There was a trend toward infants in the intervention group to be heavier at the end of the study period
(5535 ⫾ 766 g) compared with those in the control
group (5042 ⫾ 967 g; P ⫽ .07). All statistical models
controlled for gender and birth weight strata (750 –1250 g or 1251–1800 g). As far as we are aware, this is the first randomized, controlled trial conducted to evaluate the impact of adding energy and multiple nutrients to hu-man milk fed to LBW infants after discharge.
The growth results for human milk–fed infants re-ported herein are consistent with observations of higher growth among LBW infants fed nutrient-enriched as opposed to a standard term formula after hospital dis-charge in most24,28–35but not all studies.36,37For example, Carver et al24reported that infants (born⬍1800 g) who were fed a nutrient-enriched postdischarge formula (eg, 22 kcal/fl oz [3100 kJ/L], 19 g/L protein) weighed more and gained more weight until 2 months’ CA and were longer at 3 months’ CA compared with infants who were fed a standard term formula (20 kcal/fl oz [2800 kJ/L], 14 g/L protein). As was the case with human milk–fed infants in this study, infants in the study by Carver et al
who had birth weightsⱕ1250 g and were fed
nutrient-enriched feedings had larger head circumferences than LBW infants who were fed a standard term formula after hospital discharge.
Although published comparisons of infants who were fed standard term versus nutrient-enriched formula af-ter hospital discharge differed considerably in experi-mental design, growth differences between feeding
reg-imens most consistently occurred within the first few weeks of hospital discharge and among very low birth weight (VLBW) infants.11,24,32,38 This likely reflects the time frame and population at highest risk for malnutri-tion and that feeding strategies designed for term-born infants are probably insufficient to address nutrient def-icits for VLBW formula-fed infants early after discharge. We know from our previous work with a large cohort of
LBW preterm infants (n ⫽ 463) that human milk–fed
infants may, in fact, be at increased nutritional risk at hospital discharge compared with their formula-fed counterparts.9
Estimated Energy and Nutrient Intakes
The estimated energy intakes (kcal/kg per day [kJ/kg per day]) of infants in our study did not differ between feeding groups, suggesting that human milk–fed LBW infants are able to compensate to some degree for the energy and/or nutrient density of their feeding. These observations are consistent with previously published comparisons among LBW infants who were fed standard term or nutrient-enriched formula after hospital dis-charge in which no differences in energy intake were observed.24,28,31 Furthermore, these data are consistent with that of a well-controlled study in which term-born infants exhibited an early capacity to upregulate intake in response to the energy density of formula provided.39 Although energy intakes of infants in both feeding groups in this study were generally just at or below those recommended, they are consistent with those reported
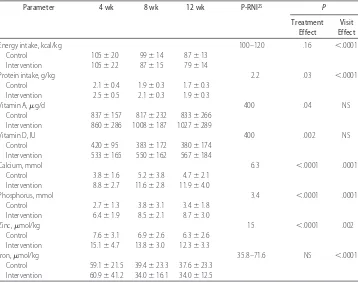
TABLE 6 Daily Energy and Select Nutrient Intake at 4, 8, and 12 Weeks After Hospital Discharge
Parameter 4 wk 8 wk 12 wk P-RNI25 P
Treatment Effect
Visit Effect
Energy intake, kcal/kg 100–120 .16 ⬍.0001
Control 105⫾20 99⫾14 87⫾13 Intervention 105⫾22 87⫾15 79⫾14
Protein intake, g/kg 2.2 .03 ⬍.0001
Control 2.1⫾0.4 1.9⫾0.3 1.7⫾0.3 Intervention 2.5⫾0.5 2.1⫾0.3 1.9⫾0.3
Vitamin A,g/d 400 .04 NS
Control 837⫾157 817⫾232 833⫾266 Intervention 860⫾286 1008⫾187 1027⫾289
Vitamin D, IU 400 .002 NS
Control 420⫾95 383⫾172 380⫾174 Intervention 533⫾165 550⫾162 567⫾184
Calcium, mmol 6.3 ⬍.0001 .0001
Control 3.8⫾1.6 5.2⫾3.8 4.7⫾2.1 Intervention 8.8⫾2.7 11.6⫾2.8 11.9⫾4.0
Phosphorus, mmol 3.4 ⬍.0001 .0001
Control 2.7⫾1.3 3.8⫾3.1 3.4⫾1.8 Intervention 6.4⫾1.9 8.5⫾2.1 8.7⫾3.0
Zinc,mol/kg 15 ⬍.0001 .002
Control 7.6⫾3.1 6.9⫾2.6 6.3⫾2.6 Intervention 15.1⫾4.7 13.8⫾3.0 12.3⫾3.3
Iron,mol/kg 35.8–71.6 NS ⬍.0001
Control 59.1⫾21.5 39.4⫾23.3 37.6⫾23.3 Intervention 60.9⫾41.2 34.0⫾16.1 34.0⫾12.5
by others who used a test-weighing procedure to mea-sure human milk intake or weighed feeding bottles to estimate formula intake.16,29,31
Mean intakes of protein, calcium, phosphorus, vita-min A, vitavita-min D, and zinc differed between infants who were fed human milk that contained extra nutrients and those who were fed human milk alone. These data sug-gest that although infants fed human milk alone may be able to upregulate their intake to match the energy intake of infants in the intervention group, they were unable to compensate for the disproportionate concen-tration of several nutrients. Given that the intakes of protein, calcium, and zinc not only differed between feeding groups but also were consumed below recom-mended levels in the control group, we speculate that these nutrients in particular may be responsible, in part, for our observations of improved length and head
cir-cumference gains (cm/wk) among infants ⱕ1250 g in
the intervention group immediately after hospital dis-charge. The lack of difference in weight gains (g/kg per day) may reflect the lack of a difference in energy intake. Our intake data are consistent with the results from studies that demonstrated improved protein, vitamin D, calcium, phosphorus, and zinc intakes among infants who consumed nutrient-enriched formula compared with those who consumed standard term formula or human milk alone after hospital discharge.24,28–31,35Lucas et al39suggested that the higher calcium and phosphorus intakes among infants who consumed nutrient-enriched formula may have contributed to the observed increase in linear growth up to 9 months’ CA in their study. Brunton et al29attributed the greater linear growth ve-locity and lean body mass accretion among infants who had bronchopulmonary dysplasia and were fed nutrient-enriched formula to higher protein and zinc intakes. Finally Wauben et al16 speculated that the higher per-centage of body fat among LBW infants who were fed human milk after hospital discharge compared with in-fant formula (by parental choice) may have been attrib-utable to lower calcium, phosphorus, and protein in-takes.
As we extensively describe elsewhere, there are a number of approaches that could be used to provide extra nutrients to human milk–fed infants after dis-charge, each with its own set of strengths and
limita-tions.2One advantage of using a powdered human milk
fortifier is that it minimizes the dilution of human milk. Limitations of using a powdered human milk fortifier include that they are not sterile and that these products were never designed or studied for use after hospital discharge. Unless carefully planned, using a powdered human milk fortifier to nutrient-enrich human milk after hospital discharge could result in inappropriately high intakes of certain nutrients, such as iron, vitamin A, and vitamin D. It is very important to note that different human milk fortifiers vary considerably in their nutrient content and are not interchangeable. To address some of the potential shortcomings of using the human milk fortifier that was used in this study (Similac Human Milk Fortifier), we altered the type and quantity of vitamin and mineral drops typically provided to human milk–fed
LBW infants at discharge. Because the human milk for-tifier that was used in this study was low in iron, infants were provided with iron drops (15 mg/day elemental iron) after hospital discharge, regardless of their feeding assignment. Instead of providing drops that contained vitamins A, D (400 IU), and C as we did in the control group, infants in the intervention group were given half the dosage of vitamin D (200 IU) and no vitamins A and C. Nutrient intakes from all sources in the intervention group were well below the lowest observed adverse effect level for vitamin A (6000 g/day) and iron (40 mg/day) and below the no observed adverse effect level for vitamin D (1800 IU/day).26,27 Although the afore-mentioned adverse effect level cutoffs were established for healthy term-born infants, these data do suggest that adding this particular powdered human milk fortifier to approximately half of expressed human milk for a finite period of time after hospital discharge can be done in such a manner so as not to provide inappropriately high levels of select nutrients.
Impact of the Intervention on Human Milk Feeding
During the feeding intervention, we saw no differences between groups with respect to the number of infants who were being human milk fed, the total volume of human milk provided each day, or the percentage of daily feedings provided as human milk. At 12 weeks
after discharge, 71 ⫾ 38% and 88 ⫾ 15.4% of daily
feedings in the control and intervention groups, respec-tively, were provided as human milk. The percentage of infants who were still being fed human milk 12 weeks after discharge in this study is much higher than that reported in the literature and is likely because they were predominantly human milk fed at discharge and because of the significant amount of lactation support that they received at home.41–44
Clinical Application
CONCLUSIONS
Results from this pilot study suggest that adding a multi-nutrient fortifier to approximately half of the milk that was fed to predominantly human milk–fed infants for 12 weeks after hospital discharge may be an effective strat-egy in addressing early discharge nutrient deficits and poor growth without unduly influencing human milk feeding when intensive lactation support is provided. In addition to the short-term growth and nutrient intake outcomes reported herein, future larger studies to eval-uate the success or failure of this feeding intervention need to include an assessment of human milk feeding rates at 1 year and longer term developmental and body composition outcomes.
ACKNOWLEDGMENTS
This study was supported by the Institute of Musculo-skeletal Health and Arthritis, Canadian Institute of Health Research. Ms Khan and Ms Weishuhn received graduate student stipends from the Canadian Institute of Health Research Training Program in Clinical Nutrition Research.
The Postdischarge Feeding Study Group also included Kirsten Kotsopoulos (Mount Sinai Hospital), Kirsten McFadden and Pauline Darling (St Michael’s Hospital), Andrea Nash (Sunnybrook Hospital), Debby Arts-Rodas (St Joseph’s Health Care), Sandra Gabriele and Jaimie MacKinnon (Credit Valley Hospital), Peter Azzopardi (Scarborough Hospitals), and Jelena Popovic (Toronto East General).
We grateful to all of the families who participated in this study and especially thank the dietitians, nurses, and physicians who assisted with study recruitment at each site. We also thank Abbott Nutrition (Montreal, Quebec, Canada) and Mead Johnson Nutritionals (Ottawa, On-tario, Canada) for providing, at our request, the human milk fortifier and many of the disposable supplies used to collect and store human milk in this study.
REFERENCES
1. Ehrenkranz RA, Younes N, Lemons JA, et al. Longitudinal growth of hospitalized very low birth weight infants.Pediatrics.
1999;104(2 pt 1):280 –289
2. O’Connor DL, Merko S, Brennan J. Human milk feeding of very low birth weight infants during initial hospitalization and after discharge.Nutr Today.2004;39(3):102–111
3. Griffin IJ. Postdischarge nutrition for high risk neonates.Clin
Perinatol.2002;29(2):327–344
4. Carlson SE. Feeding after discharge: growth, development and long-term effects. In: Tsang RC, Uauy R, Koletzko B, Zlotkin SH, eds.Nutrition of the Preterm Infant: Scientific Basis and Practical Guide-lines. 2nd ed. Cincinnati, OH: Digital Educational Publishing, Inc; 2005:357–381
5. Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current rec-ommendations in preterm infants? Pediatrics. 2001;107(2): 270 –273
6. Lemons JA, Bauer CR, Oh W, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996.Pediatrics.2001;107(1). Available at: www.pediatrics.org/cgi/content/full/107/1/e1
7. Lucas A. Nutrition, growth and development of postdischarge preterm infants. In: Silverman E, ed.Posthospital Nutrition in the
Preterm Infant. Columbus, OH: Ross Products Division, Abbott
Laboratories; 1996:81– 89
8. Merko S, Shah PS, Wong KY, et al. Nutrient intakes and growth of very preterm infants born ⬍28 weeks gestation [abstract].Can J Diet Pract Res.2002;63(2 Suppl):105
9. O’Connor DL, Jacobs J, Hall R, et al. Growth and development of premature infants fed predominantly human milk, predom-inantly premature infant formula, or a combination of human milk and premature formula.J Pediatr Gastroenterol Nutr.2003; 37(4):437– 446
10. American Academy of Pediatrics. Nutritional needs of the pre-term infant. In: Klienman R, ed.Pediatric Nutrition Handbook. 5th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2004:23–54
11. Aggett PJ, Agostoni C, Axelsson I, et al. Feeding preterm in-fants after hospital discharge: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2006; 42(5):596 – 603
12. Greer FR. Feeding the preterm infant after hospital discharge.
Pediatr Ann.2001;30(11):658 – 665
13. Schanler RJ. Post-discharge nutrition for the preterm infant.
Acta Paediatr Suppl.2005;94(449):68 –73
14. Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the use of human milk.Pediatrics.2005;115(2):496 –506 15. Canadian Institute of Health Research, Natural Sciences and
En-gineering Research Council of Canada, Social Sciences and Hu-manities Research Council of Canada.Tri-council Policy Statement: Ethical Conduct for Research Involving Humans. Ottawa, Ontario, Canada: Public Works and Government Services; 1998 16. Wauben IP, Atkinson SA, Shah JK, Paes B. Growth and body
composition of preterm infants: influence of nutrient fortifica-tion of mother’s milk in hospital and breastfeeding post-hospital discharge.Acta Paediatr.1998;87(7):780 –785 17. Casey PH, Kraemer HC, Bernbaum J, et al. Growth patterns of
low birth weight preterm infants: a longitudinal analysis of a large, varied sample.J Pediatr.1990;117(2 pt 1):298 –307 18. Casey PH, Kraemer HC, Bernbaum J, Yogman MW, Sells JC.
Growth status and growth rates of a varied sample of low birth weight, preterm infants: a longitudinal cohort from birth to three years of age.J Pediatr.1991;119(4):599 – 605
19. Gibson R, ed.Principles of Nutritional Assessment. 2nd ed. New York, NY: Oxford University Press; 2005
20. Woolridge M, Butte N, Dewey K, et al. Methods for the measure-ment of milk volume intake of the breast-fed infant. In: Jensen G, Neville MC, eds.Human Milk: Milk Components and Methodologies. New York, NY: Plenum Publishing Corp; 1985:5–21
21. Health Canada. Food Program. Canadian nutrient file, 2001. Available at: www.hc-sc.gc.ca/food-ailment/ns-sc/nr-rn/ surveillance/cnf-fcen/e_cnf_downloads.html. Accessed Febru-ary 8, 2008
22. Anderson D. Nutritional implications of premature birth, birth weight and gestational age classification. In: Groh-Wargo S, ed.
Nutritional Care for High-Risk Newborns. 3rd ed. Chicago, IL: Precept Press, Inc; 2000:3–10
23. da Silva OP, Knoppert DC. Domperidone for lactating women.
CMAJ.2004;171(7):725–726
24. Carver JD, Wu PY, Hall RT, et al. Growth of preterm infants fed nutrient-enriched or term formula after hospital discharge.
Pediatrics.2001;107(4):683– 689
26. Institute of Medicine. Vitamin D. In:Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. Wash-ington, DC: National Academy Press; 1997:250 –287
27. Institute of Medicine. Vitamin A. In:Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc. Washington, DC: National Academy Press; 2001:82–161 28. Atkinson S. Randomized trial of feeding nutrient-enriched vs
standard formula to premature infants during the first year of life [abstract].Pediatr Res.1999;45(Suppl):276A
29. Brunton JA, Saigal S, Atkinson SA. Growth and body compo-sition in infants with bronchopulmonary dysplasia up to 3 months corrected age: a randomized trial of a high-energy nutrient-enriched formula fed after hospital discharge.J Pedi-atr.1998;133(3):340 –345
30. Chan GM. Growth and bone mineral status of discharged very low birth weight infants fed different formulas or human milk.
J Pediatr.1993;123(3):439 – 443
31. Cooke RJ, Griffin IJ, McCormick K, et al. Feeding preterm infants after hospital discharge: effect of dietary manipulation on nutrient intake and growth. Pediatr Res. 1998;43(3): 355–360
32. Lucas A, Fewtrell MS, Morley R, et al. Randomized trial of nutrient-enriched formula versus standard formula for post-discharge preterm infants.Pediatrics.2001;108(3):703–711 33. Wheeler RE, Hall RT. Feeding of premature infant formula
after hospital discharge of infants weighing less than 1800 grams at birth.J Perinatol.1996;16(2 pt 1):111–116
34. Worrell LA, Thorp JW, Tucker R, et al. The effects of the introduction of a high-nutrient transitional formula on growth and development of very-low-birth-weight infants.J Perinatol.
2002;22(2):112–119
35. Lapillonne A, Salle BL, Glorieux FH, Claris O. Bone mineral-ization and growth are enhanced in preterm infants fed an isocaloric, nutrient-enriched preterm formula through term.
Am J Clin Nutr.2004;80(6):1595–1603
36. De Curtis M, Pieltain C, Rigo J. Body composition in preterm
infants fed standard term or enriched formula after hospital discharge.Eur J Nutr.2002;41(4):177–182
37. Koo WW, Hockman EM. Posthospital discharge feeding for preterm infants: effects of standard compared with enriched milk formula on growth, bone mass, and body composition.
Am J Clin Nutr.2006;84(6):1357–1364
38. Cooke RJ, Embleton ND, Griffin IJ, Wells JC, McCormick KP. Feeding preterm infants after hospital discharge: growth and de-velopment at 18 months of age.Pediatr Res.2001;49(5):719 –722 39. Fomon SJ, Filmer LJ Jr, Thomas LN, Anderson TA, Nelson SE. Influence of formula concentration on caloric intake and growth of normal infants.Acta Paediatr Scand.1975;64(2):172–181 40. Lucas A, Bishop NJ, King FJ, Cole TJ. Randomised trial of
nutrition for preterm infants after discharge. Arch Dis Child.
1992;67(3):324 –327
41. Callen J, Pinelli J. A review of the literature examining the benefits and challenges, incidence and duration, and barriers to breastfeeding in preterm infants.Adv Neonatal Care.2005;5(2): 72– 88, quiz 89 –92
42. Furman L, Minich N, Hack M. Correlates of lactation in moth-ers of very low birth weight infants.Pediatrics.2002;109(4). Available at: www.pediatrics.org/cgi/content/full/109/4/e57 43. Kaufman KJ, Hall LA. Influences of the social network on
choice and duration of breast-feeding in mothers of preterm infants.Res Nurs Health.1989;12(3):149 –159
44. Pinelli J, Atkinson SA, Saigal S. Randomized trial of breast-feeding support in very low-birth-weight infants.Arch Pediatr
Adolesc Med.2001;155(5):548 –553
45. Institute of Medicine. Vitamin E. In:Dietary Reference Intakes For Vitamin C, Vitamin E, Selenium and Carotenoids. Washington, DC: National Academy Press; 2000:186 –283
46. Atkinson S. Effects of gestational stage at delivery on human milk components. In: Jensen R, ed.Handbook of Milk Composi-tion. San Diego, CA: Academic Press; 1995:222–237
47. Klein CJ. Nutrient requirements for preterm infant formulas.J
Nutr.2002;132(6 Suppl 1):1395S–1577S
LURING DOCTORS AND NURSES “A CRIME”
“Rich countries are actively poaching so many African health workers that the practice should be viewed as a crime, a team of international disease
experts say in the British medical journal The Lancet. More than 13 000
doctors trained in sub-Saharan Africa are now practicing in Britain, the United States, Canada and Australia, leaving behind colleagues struggling to cope with impossible caseloads. African nurses and pharmacists are also sought after by clinics, hospitals and drug store chains offering better pay and legal assistance with immigration, said the experts, who include the heads of several schools of pharmacy or medicine in African countries. ‘‘The resulting dilapidation of health infrastructure contributes to a measurable and foresee-able public health crisis,’ the article said. ’’The practice should therefore be viewed as an international crime.’”
Reuters.New York Times. February 22, 2008
DOI: 10.1542/peds.2007-0054
2008;121;766-776
Pediatrics
Carol Westall, Hilary Whyte and on behalf of the Postdischarge Feeding Study Group
Jefferies, Douglas M. Campbell, Elizabeth Asztalos, Mark Feldman, Joanne Rovet,
Deborah L. O'Connor, Sobia Khan, Karen Weishuhn, Jennifer Vaughan, Ann
With Extra Energy and Nutrients After Hospital Discharge
Growth and Nutrient Intakes of Human Milk Fed Preterm Infants Provided
& Services
Updated Information
http://www.pediatrics.org/cgi/content/full/121/4/766
including high-resolution figures, can be found at:
References
http://www.pediatrics.org/cgi/content/full/121/4/766#BIBL
at:
This article cites 33 articles, 12 of which you can access for free
Citations
s
http://www.pediatrics.org/cgi/content/full/121/4/766#otherarticle
This article has been cited by 2 HighWire-hosted articles:
Subspecialty Collections
n
http://www.pediatrics.org/cgi/collection/premature_and_newbor Premature & Newborn
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.pediatrics.org/misc/Permissions.shtml
tables) or in its entirety can be found online at:
Information about reproducing this article in parts (figures,
Reprints
http://www.pediatrics.org/misc/reprints.shtml