Effects of Pseudomonas fluorescens and
fertilizers on the reproduction of
Meloidogyne incognita and growth of tomato
Zaki A. Siddiqui
∗, Arshid Iqbal, Irshad Mahmood
Department of Botany, Aligarh Muslim University, Aligarh 202002, IndiaReceived 6 September 1999; received in revised form 10 May 2000; accepted 10 May 2000
Abstract
A 60-day glasshouse experiment was conducted to assess the influence of two strains of Pseudomonas fluorescens (GRP3 and PRS9), organic manure, and inorganic fertilizers (urea, diammonium phosphate (DAP), muriate of potash and monocalcium phosphate) alone and in combination on the multiplication of Meloidogyne incognita and growth of tomato. Pseudomonas
fluorescens GRP3 was better at improving tomato growth and reducing galling and nematode multiplication than PRS9.
Organic manuring resulted in less galling and nematode multiplication than occurred with DAP. However, DAP was found better in reducing nematode multiplication and improving plant growth than urea. Muriate of potash was the inorganic fertilizer least effective in reducing galling and nematode multiplication. Pseudomonas fluorescens GRP3 with organic manure was the best combination for the management of M. incognita on tomato but improved management of M. incognita can also be obtained if DAP is used with the GRP3 strain of P. fluorescens. © 2001 Elsevier Science B.V. All rights reserved.
Keywords: Fertilizers; Management; Meloidogyne; Pseudomonas; Tomato
1. Introduction
Nematodes cause about 20.6% worldwide yield loss (Sasser, 1989). Yield losses in India due to the root-knot nematodes (Meloidogyne spp.) range from 39.7 to 46.0% (Bhatti and Jain, 1977; Reddy, 1985). Plants infected with Meloidogyne spp. show stunt-ing and the typical symptoms of root gallstunt-ing. Some infected plants exhibit nutrient deficiency symp-toms, particularly nitrogen. Tomato, Lycopersicon
esculentum Mill. is an important vegetable crop and
is parasitized by root-knot nematodes.
∗Corresponding author. Tel.:+91-571-404398; fax:+91-571-401202.
Rhizosphere microorganisms may provide defence against pathogen attack (Weller, 1988). The rhizoplane and rhizosphere are colonized or otherwise occupied by many microorganisms and plant growth promoting bacteria are capable of providing substantial protec-tion against nematode diseases (Siddiqui and Mah-mood, 1999). Similarly, organic manure may suppress plant parasitic nematode populations and improve crop tolerance (Southey, 1978). Nematode-infected plants generally show foliar symptoms of nutrient deficiency (Birat, 1963; Good, 1968) and it has been reported that manipulation of fertilizer may influence nematode development and reproduction (Pant et al., 1983; Zaki and Bhatti, 1989; Akhtar et al., 1998).
In the present study, an attempt was made to use
Pseudomonas fluorescens (strains GRP3 and PRS9),
organic manure and inorganic fertilizers, namely urea, diammonium phosphate (DAP), muriate of potash and monocalcium phosphate, for the management of
Meloidogyne incognita on tomato.
2. Materials and methods
The root-knot nematode M. incognita (Kofoid & White) Chitwood was the test pathogen. Two strains of P. fluorescens, viz. GRP3 and PRS9, or-ganic manure, and inoror-ganic fertilizers (urea, DAP, muriate of potash and monocalcium phosphate) were applied alone and in combination to tomato (L. esculentum cv. Pusa Ruby). The influence of treatments on plant growth, galling and nematode reproduction were assessed in a 60-day glasshouse experiment.
2.1. Preparation and sterilization of soil mixture
Sandy loam soil collected from a field belonging to the Botany Department, AMU., Aligarh was passed
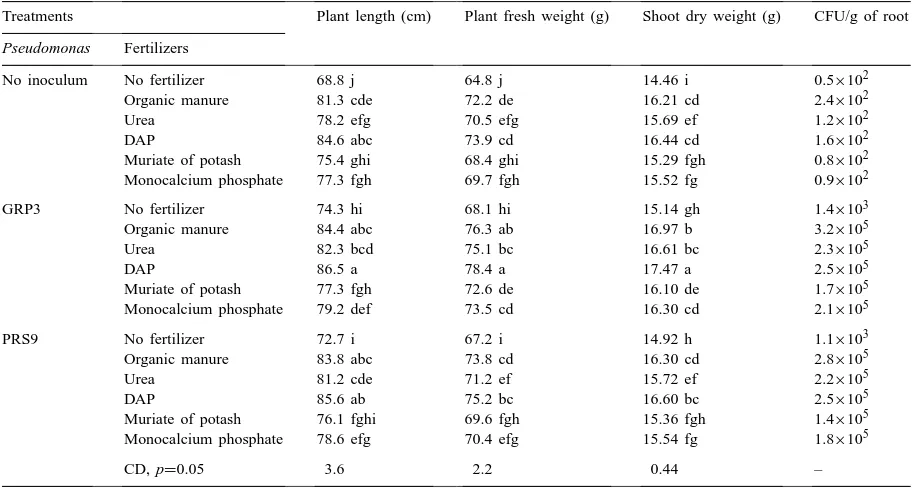
Table 1
Effect of two strains of Pseudomonas fluorescens and fertilizers on the growth of tomatoa
Treatments Plant length (cm) Plant fresh weight (g) Shoot dry weight (g) CFU/g of root
Pseudomonas Fertilizers
No inoculum No fertilizer 68.8 j 64.8 j 14.46 i 0.5×102
Organic manure 81.3 cde 72.2 de 16.21 cd 2.4×102
Urea 78.2 efg 70.5 efg 15.69 ef 1.2×102
DAP 84.6 abc 73.9 cd 16.44 cd 1.6×102
Muriate of potash 75.4 ghi 68.4 ghi 15.29 fgh 0.8×102
Monocalcium phosphate 77.3 fgh 69.7 fgh 15.52 fg 0.9×102
GRP3 No fertilizer 74.3 hi 68.1 hi 15.14 gh 1.4×103
Organic manure 84.4 abc 76.3 ab 16.97 b 3.2×105
Urea 82.3 bcd 75.1 bc 16.61 bc 2.3×105
DAP 86.5 a 78.4 a 17.47 a 2.5×105
Muriate of potash 77.3 fgh 72.6 de 16.10 de 1.7×105
Monocalcium phosphate 79.2 def 73.5 cd 16.30 cd 2.1×105
PRS9 No fertilizer 72.7 i 67.2 i 14.92 h 1.1×103
Organic manure 83.8 abc 73.8 cd 16.30 cd 2.8×105
Urea 81.2 cde 71.2 ef 15.72 ef 2.2×105
DAP 85.6 ab 75.2 bc 16.60 bc 2.5×105
Muriate of potash 76.1 fghi 69.6 fgh 15.36 fgh 1.4×105 Monocalcium phosphate 78.6 efg 70.4 efg 15.54 fg 1.8×105
CD, p=0.05 3.6 2.2 0.44 –
aDifferent letters represent values within columns that are significantly different at p≤0.05 based on the Duncan’s multiple range test.
through a 10 mesh sieve. The soil, river sand and organic manure (decomposed cow dung) were mixed in the ratio of 3:1:1, and 15 cm diameter clay pots were each filled with 1 kg of the mixture. A lit-tle water was poured into each pot to just wet the soil surface before transferring them to an autoclave for sterilization at 137.9 kPa for 20 min. Sterilized pots were allowed to cool at room temperature before use.
2.2. Raising and maintenance of the test plant
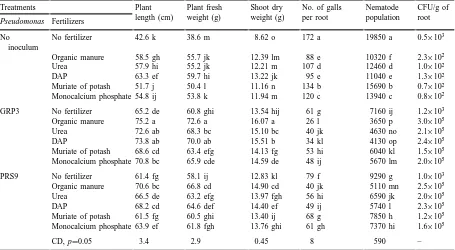
Table 2
Effect of two strains of Pseudomonas fluorescens and fertilizers on the reproduction of Meloidogyne incognita and growth of tomatoa
Treatments Plant
length (cm)
Plant fresh weight (g)
Shoot dry weight (g)
No. of galls per root
Nematode population
CFU/g of root Pseudomonas Fertilizers
No inoculum
No fertilizer 42.6 k 38.6 m 8.62 o 172 a 19850 a 0.5×103
Organic manure 58.5 gh 55.7 jk 12.39 lm 88 e 10320 f 2.3×102
Urea 57.9 hi 55.2 jk 12.21 m 107 d 12460 d 1.0×102
DAP 63.3 ef 59.7 hi 13.22 jk 95 e 11040 e 1.3×102
Muriate of potash 51.7 j 50.4 l 11.16 n 134 b 15690 b 0.7×102 Monocalcium phosphate 54.8 ij 53.8 k 11.94 m 120 c 13940 c 0.8×102
GRP3 No fertilizer 65.2 de 60.8 ghi 13.54 hij 61 g 7160 ij 1.2×103
Organic manure 75.2 a 72.6 a 16.07 a 26 l 3650 p 3.0×105
Urea 72.6 ab 68.3 bc 15.10 bc 40 jk 4630 no 2.1×105
DAP 73.8 ab 70.0 ab 15.51 b 34 kl 4130 op 2.4×105
Muriate of potash 68.6 cd 63.4 efg 14.13 fg 53 hi 6040 kl 1.5×105 Monocalcium phosphate 70.8 bc 65.9 cde 14.59 de 48 ij 5670 lm 2.0×105
PRS9 No fertilizer 61.4 fg 58.1 ij 12.83 kl 79 f 9290 g 1.0×103
Organic manure 70.6 bc 66.8 cd 14.90 cd 40 jk 5110 mn 2.5×105
Urea 66.5 de 63.2 efg 13.97 fgh 56 hi 6590 jk 2.0×105
DAP 68.2 cd 64.6 def 14.40 ef 49 ij 5740 l 2.3×105
Muriate of potash 61.5 fg 60.5 ghi 13.40 ij 68 g 7850 h 1.2×105 Monocalcium phosphate 63.9 ef 61.8 fgh 13.76 ghi 61 gh 7370 hi 1.6×105
CD, p=0.05 3.4 2.9 0.45 8 590 –
aDifferent letters represent values within columns that are significantly different at p=0.05 based on the Duncan’s multiple range test.
randomized block design and each treatment was replicated five times. Pots were watered as needed and the experiment was terminated 60 days after inoculation.
2.3. Preparation of nematode inoculum
Large number of M. incognita egg masses were hand picked, using sterilized forceps, from heavily infected brinjal roots on which a pure culture of the nematode was maintained. These egg masses were washed in distilled water and then placed in 10 cm diameter 15 mesh coarse sieves containing crossed layers of tissue paper and placed in Petri plates con-taining water just deep enough to contact the egg masses. The hatched juveniles were collected from the Petri plates every 24 h and fresh water was added to the Petri plates. The concentration of second stage juveniles of M. incognita in the water suspension was adjusted so that each milliliter contained 200±5 nematodes. Ten milliliters of this suspension (i.e.
2000 freshly hatched juveniles) were added to each pot containing a tomato seedling.
2.4. Bacterial inoculum
Charcoal-soil based commercial cultures of two strains of Fluorescent pseudomonads, viz. GRP3 and PRS9 were obtained from the Department of Mi-crobiology, G.B. Pant University of Agriculture and Technology, Pantnagar, U.P. One hundred grams of culture of each strain were suspended in 1000 ml distilled water and 10 ml (equivalent to 1 g of cul-ture) were added around each seedling. One gram culture of GRP3 strain had 2.6×106 viable bacterial cells while PRS9 strain had 2.7×106viable cells/g of culture.
2.5. Organic manuring
transplanting, in the pots as shown in Tables 1 and 2. Prior to use the cow dung has been allowed to decompose in a container for 1 year, with sufficient water being added at 10-day intervals. Five grams of composted organic manure contains about 15 mg N, 3.3 mg P and 12.4 mg K.
2.6. Inorganic fertilizers
Urea (Co(NH2)2), diammonium phosphate ((NH4)2
HPO4), muriate of potash (KCl) and monocalcium
phosphate (Ca(H2PO4)2) were used as inorganic
fer-tilizers. To prepare the treatments 100 g of each in-organic fertilizer was dissolved separately in 1000 ml distilled water and 10 ml of this suspension (which contain 1 g fertilizer) were added to each pot. One gram urea contains 460 mg N while 210 mg N and 230 mg P were available in the same quantity of DAP. Similarly, 1 g muriate of potash contains 498 mg K while in 1 g monocalcium phosphate, 90 mg P was available.
2.7. Inoculation technique
For inoculation of M. incognita, and the Fluo-rescent pseudomonads, and the addition of organic manure and inorganic fertilizers, soil around the roots was carefully moved aside without damaging the roots. The inoculum suspensions, fertilizer so-lutions and compost were poured or placed around the roots and the soil replaced. In the control treat-ments where no bacterial inoculum and no fertilizer was applied, water was added in equal volume to the inoculum suspension. The bacterial and manurial treatments were applied as shown in Table 1 (with-out M. incognita) and Table 2 (with nematodes). In each case there were 18 treatments comprising three treatments of Pseudomonas (no Pseudomonas, GRP3 and PRS9=factor 1), each tested with six fertilizer treatments (no fertilizer, organic manure, urea, DAP, muriate of potash and monocalcium=
factor 2).
2.8. Observations
Plants were uprooted 60 days after inoculation and the root systems were gently rinsed. The plants were
cut with a knife above the base of the root emergence zone, and the length of shoots and roots were recorded in cm from the cut end to the top of the first leaf and the longest root, respectively. Excess water was removed by blotting before weighing shoots and roots separately. The number of galls per root system was counted. For dry weight determination, shoots were kept in envelopes at 60◦C for 2–3 days.
A 250 g sub-sample of well mixed soil from each treatment was processed by Cobb sieving and decant-ing followed by Baermann funnel extraction. Nema-tode suspensions were collected after 24 h, and the numbers of nematodes were counted in five aliquots of 1 ml of suspension from each sample. The means of the five counts were used to calculate the population of nematodes per kg soil. To estimate the number of juve-niles, eggs and females inside the roots, 1 g sub-sample of root was macerated for 30–40 s in a Waring blender and counts were made on the suspension thus obtained. Total numbers of nematodes present in the roots were calculated by multiplying the number of nematodes present in 1 g of root by the total weight of root. The sizes of the galls were also measured in the different treatments and histopathological study of galls from different treatments permitted differences in size of gi-ant cells to be observed. For histopathological studies of nematodes, infected roots from different treatments were embedded in wax and sectioned using a micro-tome and observations of giant cells were made under a microscope.
To isolate bacteria from roots, 1 g root samples were rinsed with tap water and homogenized in a known amount of sterile distilled water. A serial dilution of this root suspension was plated on to nutrient agar to observe the growth of P. fluorescens. The plates were incubated for 24 h at 37◦C and the total number of colonies formed in 1 g of root was calculated from the serial dilutions and presented as colony forming units (CFU) per gram of root.
2.9. Statistical analysis
(factor 1 P. fluorescens and factor 2 fertilizers) on a PHA3000/400 AS system with a VMS operating system. Critical differences (CD) were calculated at
p=0.05 and Duncan’s multiple range test was em-ployed to test for significant differences between treatments.
3. Results
Analysis as a single three factor experiment indicate that the effects of Pseudomonas, fertilizer and nema-todes on plant growth were all significant (p<0.05). The interaction effects of Pseudomonas×fertilizer,
Pseudomonas×nematodes, nematodes×fertilizer and
Pseudomonas×fertilizer×nematode were also
sig-nificant. The effects of Pseudomonas and fertilizer and also their interaction on galling and nematode multiplication were significant.
Significant increases in the growth of tomato plants were observed when plants without nematodes were treated with either strain of P. fluorescens, organic manure or inorganic fertilizer (Table 1). GRP3 and PRS9 applied in combination with organic manure and inorganic fertilizer to plants without nematodes had similar effects on plant growth. The use of DAP or organic manure with GRP3 was significantly better than GRP3 with monocalcium phosphate or muri-ate of potash but GRP3 with urea was not signifi-cantly better than GRP3 with monocalcium phosphate (Table 1).
Treatments with P. fluorescens, organic manure and inorganic fertilizers all resulted in significantly increased plant growth of nematode-inoculated plants when compared with nematode-inoculated untreated plants (Table 2). Of the single treatments
Pseu-domonas GRP3 and DAP caused the greatest
in-creases in the growth of nematode-inoculated plants, while PRS9 was more effective in improving growth of nematode-inoculated plants than organic manure, urea or monocalcium phosphate. Muriate of potash was the least effective treatment in improving growth of nematode infected plants. Organic manure with P.
fluorescens GRP3, caused the greatest increase of all
treatment combination in the growth of nematode in-fected plants (based on shoot dry weight). DAP with GRP3 was not significantly better than GRP3 plus urea. GRP3 with muriate of potash was significantly
less effective than GRP3 plus urea in improving growth of nematode-inoculated plants, but not signif-icantly less effective than GRP3 plus monocalcium phosphate. Although PRS9 with organic manure or inorganic fertilizers caused significant improvement in plant growth, the results were less noticed than where GRP3 was used with organic manure and inorganic fertilizers (Table 2).
Greater reductions in galling and nematode multi-plication were observed when plants were inoculated with the GRP3 strain of P. fluorescens than in any other single treatment, followed by PRS9, organic manure, DAP, urea, monocalcium phosphate and muriate of potash (Table 2). The greatest reduction in galling and nematode multiplication was observed when the GRP3 strain of P. fluorescens was used with organic manure. DAP with GRP3 caused sta-tistically similar reduction in galling and nematode multiplication to that caused by urea plus GRP3. Muriate of potash with GRP3 was less effective in reducing galling and nematode multiplication but not significantly worse than GRP3 when used with monocalcium phosphate. All the treatments which included GRP3 permitted significantly less galling than the treatment without Pseudomonas. The com-bined use of PRS9 with organic manure or inor-ganic fertilizers was less effective in reducing galling and nematode multiplication than when GRP3 was used with organic manure or inorganic fertilizers (Table 2).
Greater root colonization by P. fluorescens was found after using the GRP3 strain than the PRS9 strain (Tables 1 and 2), although the PRS9 strain had slightly higher numbers of cells in 1 g culture than GRP3 (see Section 2.4). Use of either strain of P.
fluorescens with fertilizers resulted in greater root
4. Discussion
Both strains of fluorescent pseudomonads tested were found to increase the growth of nematode-inoculated and unnematode-inoculated plants. Plant growth promoting pseudomonads may act through direct antagonism of pathogens, antibiotic production, com-petition with pathogens for essential nutrients such as iron and, more directly through plant growth promotion (Gamliel and Katan, 1993). In addition, an induced systemic resistance by fluorescent pseu-domonads is also considered to be a mechanism for biocontrol of pathogens (Wei et al., 1996). Of the two strains used, GRP3 was more effective than PRS9 in reducing multiplication of M. incognita. The sizes of
M. incognita induced galls and giant cells were also
smaller in the roots treated with GRP3 than PRS9. The GRP3 strain was a more aggressive root colonizer than PRS9, an important feature for introduced bacteria in the management of root pathogens (Suslow, 1982).
The use of P. fluorescens with organic manure was better than the use of P. fluorescens with inorganic fertilizers. Organic manuring results in several bene-fits such as better soil structure which provides a more suitable medium for plant growth. Organic manures supply nutrients for the plant and also help to build up antagonistic organisms. The combined use of or-ganic manure with P. fluorescens resulted in the build up of high bacterial populations which probably ad-versely affected the nematode population and thereby improved plant growth. Availability of nutrients may also be helpful for bacterial colonization of roots. The nematicidal potential of inorganic fertilizers is well known (Rodriguez-Kabana et al., 1981, 1982). Urea is readily converted to ammonia by urease present in soil, a conversion which is necessary for urea to be effective both as a fertilizer and as a nematicide. It is generally believed that ammoniacal nitrogen is more damaging to nematodes than nitrate nitrogen (Badra and Khattab, 1980). DAP, which has both ammoni-acal nitrogen and phosphate was better than urea at reducing nematode populations. Besides the ammo-niacal nitrogen, phosphate present in DAP was useful in enhancing root growth and increasing host toler-ance along with root absorptive capacity (Hussey and Roncadori, 1982). Phosphate is known to reduce soil pH, which has an adverse effect on nematode multi-plication (Pant et al., 1983). Potassium was the least
effective inorganic fertilizer in reducing nematode multiplication but an inhibitory effect of potassium on nematodes was reported by Gupta and Mukhopad-hyaya (1971).
References
Akhtar, M., Siddiqui, Z.A., Mahmood, I., 1998. Management of root-knot nematode Meloidogyne javanica by some inorganic fertilizers on tomato. Nematol. Medit. 26, 23–25.
Badra, T., Khattab, M.M., 1980. The effect of nitrogen fertilizers on the growth of Olive in relation to infestation of Rotylenchulus reniformis. Nematol. Medit. 8, 67–72.
Bhatti, D.S., Jain, R.K., 1977. Estimation of loss in okra, tomato and brinjal yield due to Meloidogyne javanica. Indian J. Nematol. 7, 37–41.
Birat, R.B.S., 1963. Effect of major plant nutrient in gall formation. Sci. Cult. 29, 311–312.
Dospekhov, B.A., 1984. Field experimentation. In: Statistical Procedures. Mir Publishers, Moscow, 352 pp.
Gamliel, A., Katan, J., 1993. Suppression of major and minor pathogens by Fluorescent pseudomonads in solarized and non-solarized soil. Phytopathology 83, 68–75.
Good, J.M., 1968. Relation of plant parasitic nematodes to management practices. In: Smart, G.C., Perry, V.G. (Eds.), Tropical Nematology. University of Florida Press, Gainesville, pp.113–138.
Gupta, D.C., Mukhopadhyaya, M.C., 1971. Effect of N, P, and K on root-knot nematode Meloidogyne javanica (Treub) Chitwood. Sci. Cult. 31, 246–327.
Hussey, R.S., Roncadori, R.N., 1982. Vesicular arbuscular mycorrhizae may limit nematode activity and improve plant growth. Plant Dis. 66, 9–14.
Pant, V., Hakim, S., Saxena, S.K., 1983. Effect of different levels of N, P, K on the growth of tomato Marglobe and on the morphometrics of root-knot nematode Meloidogyne incognita. Indian J. Nematol. 13, 110–113.
Reddy, D.D.R., 1985. Analysis of crop losses in tomato due to Meloidogyne incognita. Indian J. Nematol. 15, 55–59. Rodriguez-Kabana, R., King, P.S., Pope, M.H., 1981. Combination
of anhydrous ammonia and ethylene dibromide for control of nematodes parasitic on soybean. Nematropica 11, 27–41. Rodriguez-Kabana, R., Shelby, R.A., King, P.S., Pope, M.H., 1982.
Combination of anhydrous ammonia and 1,3-dichloropropenes for control of nematodes parasitic on soybean. Nematropica 12, 61–69.
Sasser, J.N., 1989. Plant parasitic nematodes: the farmer’s hidden enemy. A cooperative publication of the Department of Plant Pathology and Consortium for International Crop Protection. 115 pp.
Siddiqui, Z.A., Mahmood, I., 1999. Role of bacteria in the management of plant parasitic nematodes: a review. Biores. Technol. 69, 167–179.
Suslow, T.V., 1982. Role of root colonizing bacteria in plant growth. In: Mount, M.S., Lacy, G.H. (Eds.), Phytopathogenic Prokaryotes, Vol. 1. Academic Press, London, pp. 187–223. Wei, G., Kolepper, J.W., Tuzum, S., 1996. Induced systemic
resistance to encounter diseases and increased plant growth by plant growth promoting bacteria under field conditions. Phytopathology 86, 221–224.
Weller, D.M., 1988. Biological control of soil borne plant pathogens in the rhizosphere with bacteria. Ann. Rev. Phytopathol. 26, 379–407.