Cell walls harbor proteins and polysaccharides able to
condition the development of a plant. In the past year, genes
and enzymes modulating the composition and physical
properties of walls have been characterized, and wall
composition has been linked to the way a cell interacts with
another cell, and to the way in which it differentiates. The sum
of the signaling and physical activities of a cell wall may explain
much about the control of development.
Addresses
Ceres Inc., 3008 Malibu Canyon, Malibu, California 90265, USA; e-mail: [email protected]
Current Opinion in Plant Biology1998, 1:504–510
http://biomednet.com/elecref/1369526600100504
© Current Biology Ltd ISSN 1369-5266
Abbreviations
AGP arabinogalactan-protein HRGP hydroxyproline-rich glycoprotein Mab monoclonal antibody
Introduction
Most cell divisions in plants take place in meristems, and
new cells choose from a variety of potential fate. The
divi-sions generate the correct numbers of cells, and pattern
formation sets up the anatomy. Progression through the
cell cycle seems to require the accurate assembly of a cell
wall. Signaling between newly divided cells then directs
the choice of developmental pathway and some of these
signals are thought to arise from the wall itself [1].
A cell differentiates some time after it has progressed
through a developmental pathway. This differentiation
process usually involves an up to one thousand-fold
increase in cell volume. The enlargement is brought about
by the relaxation of the wall and turgor-driven wall
expan-sion, followed by the re-rigidification and changes in wall
structure. Eventually, walls can be irreversibly reinforced,
either by the addition of new components to the wall or
further cross-linking of existing components [2
•].
Thus, the function of a cell is intimately linked to the
composition and structure of its wall. A comprehensive
understanding of the functions of a wall hinges on analysis
of wall assembly and metabolism. To this end, analysis of
wall polymers has revealed novel aspects of wall structure,
and these have been complemented by screens for plants
defective in wall biosynthesis. Experiments have shown
that a cell identified by its wall can control the fate of
another cell, and that walls are involved in cell–cell
inter-actions; these and other studies suggest that signal
molecules released from walls are involved with these
developmental processes
in planta
. The localization of
genes and proteins affecting wall relaxation and the
chem-ical analysis of wall cross-linking have helped to resolve
the mechanism of cell enlargement and rigidification.
The control of the cell cycle is a key regulator of plant
morphogenesis [3] and it is well documented that cells in
different stages of the cell cycle have characteristic walls.
For example, higher levels of unesterified pectins have
been localised to non-dividing cells than to dividing cells
in the root tip of
Arabidopsis
[4
•], and the expression of two
classes of hydroxyproline-rich glycoproteins (HRGPs),
extensins and arabinogalactan-proteins (AGPs), were
shown to be dependent on the stage of the cell cycle and
on cell proliferation in
Catharanthus
and carrot [5
•,6
•].
Their cell cycle functions are unknown; however, the
tomato pectin methylesterase gene
PMEU
1 was cloned
and characterized [7
•], and gain-of-function and
loss-of-function experiments with this gene may help to address
these functions directly. It was previously known that
pro-lyl hydroxylation inhibitors caused the disappearance of
HRGP from the wall and also blocked cell division. Now,
the cloning of
PsHRGP
1, which encodes an extensin
repressed in dividing pea cells [8
•], may prove useful in
studying the role of HRGPs in cell division more directly.
These findings have further demonstrated that walls play
diverse and essential roles in plant cell development.
Here, I summarize recent work on the assembly, structure,
and function of cell wall components.
Wall assembly and structure
Polysaccharides and proteins are deposited in a new wall,
and together provide the wall with its properties.
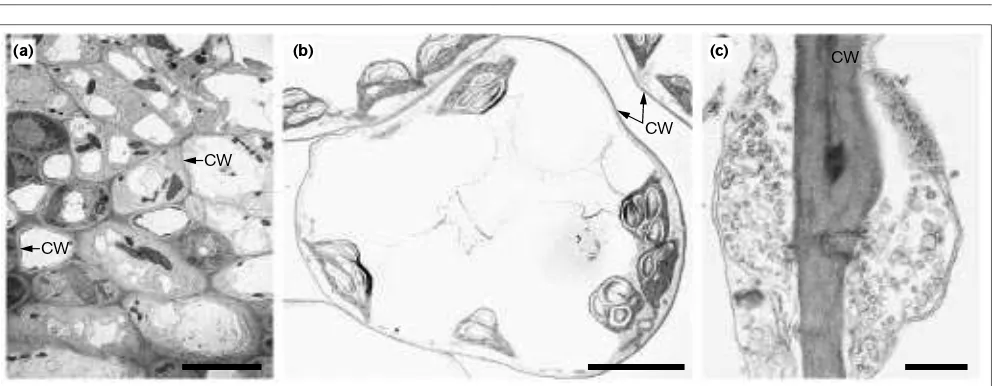
Figure 1 shows some pictures of
Arabidopsis
leaf cells and
their walls. Cellulose is the major wall polymer, but
genes active in its synthesis were only recently identified
[9]. This is due in part to difficulty in designing genetic
screens for plants deficient in cellulose. In
Arabidopsis
,
rsw1
mutations cause a reduction in cellulose, the
accu-mulation of a less-ordered
β
-1,4-glucan, and widespread
morphological abnormalities [10].
RSW
1 was cloned, and
found to encode the catalytic subunit of cellulose
syn-thase [11
••]. This confirmed the central importance of
cellulose in plant development. The demonstration that
cellulose is absent from xylem walls in
Arabidopsis irx
mutants, and that these xylem cells collapse at normal
water pressure, further suggests that cellulose may serve
as a template for the assembly of lignin [12
••], and have
a dual role in morphogenesis. The role of an E-type
endo-1,4-
β
-D-glucanase, which catalyses the breakdown
of cellulose rather than its assembly, active during the
same process in tomato [13
•], is less clear. One
possibili-ty is that this glucanase is involved with the cutting and
spacing of the cellulose.
Cell walls: structures and signals
Cellulose is hydrogen-bonded to xyloglucan. Incubation of
pea microsomes with UDP-[
14C] galactose and tamarind
seed xyloglucan allowed the identification of a galactosyl
transferase involved with xyloglucan biosynthesis [14
•].
The tobacco polygalacturonate-4-
α
-galacturonosyltrans-ferase that catalyses the transfer of UDP-[
14C] galacturonic
acid to homogalacturonan acceptor substrates was also
iso-lated and characterized [15
•]. Xyloglucan and pectin play
important roles in morphogenesis, and the manipulation of
these enzymes may facilitate an understanding of their
function. The characterization of some novel wall proteins
[16
•] could also be useful in this respect.
In a genetic approach to the analysis of wall
polysaccha-ride function, mutagenized
Arabidopsis
plants were used
to identify 11 loci involved with wall biosynthesis [17
••].
Mutations causing the absence of fucose (
mur1
),
decreased levels of fucose (
mur2
,
mur3
), arabinose (
mur4
,
mur5
,
mur6
,
mur7
), or rhamnose (
mur8
), or complex
alter-ations in the proportions of several monosaccharides
including fucose, arabinose and rhamnose (
mur9
,
mur10
,
mur11
), were identified [17
••]. Some mutations (
mur1
,
mur9
,
mur10
) resulted in dwarfism [17
••]. This showed
that cell walls are amenable to genetic analysis, and also
that changes in certain monosaccharides are associated
with alterations in development.
Signaling by walls
Local interactions between the cells in a meristem play an
important role in pattern formation in plants. Laser ablation
studies were used to demonstrate that non-dividing cells in
the quiescent centers of
Arabidopsis
roots inhibit the
differ-entiation of surrounding cells; the signals achieving this
inhibition operate within the range of a single cell [18
••].
Walls form the point of contact between cells; therefore,
one possibility is that the wall dictates this local signaling.
In the
Arabidopsis
mutant
fdh1
, the shoot epidermis is
mod-ified such that it can allow pollen grains to geminate and
enable organs that come into contact to fuse. This suggests
that a developmental program normally operating only in
carpels is activated ectopically. Wall and cuticular
perme-ability were found to be disturbed in
fdh1
mutants,
apparently as a result of altered lipid composition [19
••].
Local signaling events, therefore, could be conditioned by
wall lipid. Pollen germination on stigmaless
Petunia
styles
can be enabled by trilinolein [20
••], a triacylglyceride lipid
component of wet stigma exudates, suggesting that these
triacylglycerides could be extracellular signaling molecules.
[image:2.612.59.557.94.287.2]Unusual polysaccharide profiles seem to be characteristics
of cell walls in embryonic suspension cultures. Walls in
such cultures of pine were found to contain higher levels
of xylans, fucoxylans, and arabinogalactans than in
non-embryogenic lines [21]. Soluble signals including soluble
AGPs help to control somatic embryogenesis, so one
possi-bility is that the wall contributes to a pool of such signals.
In an embryogenic culture of carrot, some cells label at the
wall with the monoclonal antibody (Mab) JIM8, which
rec-ognizes a rhamnose-containing epitope present in certain
AGPs. JIM8-reactive cells were found to control the
devel-opment of JIM8-unreactive embryo initial cells, and
conditioned growth medium from cultures of
JIM8-reac-tive cells could substitute for the cells themselves [22
••].
This showed that cells identifiable by their wall
composi-tion release diffusible signals, possibly from their walls, to
control a developmental process [22
••]. The
transforma-tion of
Zinnia
mesophyll cells into metaxylem-like cells
was also found to be stimulated by soluble signal
mole-cules that were small and heat-resistant [23
•]. Two
tetrasaccharides of rhamnogalacturonan, differing only in
an
O
-3 acetyl group at an internal galacturonosyl residue,
activated D-glycohydrolases in cultured
Rubus
cells in
Figure 1Electron micrographs of Arabidopsisleaf cells. A thin cell wall (CW) encloses each cell. Walls are thought to be involved with cell division, cell–cell interaction, and cell differentiation. The bar in (a)= 50µm, in (b)= 10µm, and in (c)= 0.1µm.
CW
CW
CW
(a) (b) (c)
what could be a related process [24
•]. Walls and soluble
oligosaccharide signals released from them may, therefore,
be active in diverse aspects of cell development.
Labeling studies with Mabs reinforced the view that
sig-nals from walls are involved with vascular development
and cell patterning
in planta
[25
•]. For example, epitopes
carried by some AGPs were shown to occur in root apices
in patterns that were mutually exclusive, and also
reflect-ing the distribution of extensin epitopes, and both AGP
and extensin epitopes were associated with future cell
anatomy [26
•]. The epitope recognized by JIM15 was only
found on an oxidatively coupled sugar beet AGP [27
•].
Oxidative cross-linking of molecules in cells is usually
brought about by H
2O
2, suggesting that H
2O
2has a role in
AGP function [27
•].
β
-glucosyl Yariv reagent, which binds
to AGPs, caused the appearance of shootless embryos
when added to a carrot suspension culture [28
•], and
inhib-ited root elongation when added to
Arabidopsis
seedlings
[29
•]. Although
β
-glucosyl Yariv reagent was found not to
be specific for AGPs in certain circumstances [30
•], these
experiments with carrot and
Arabidopsis
featured controls
with
α
-glucosyl reagent, and support the idea that certain
AGPs have mechanical functions such as cell expansion
functions.
NaPRP
5, which codes for the core protein of a
Nicotiana
style AGP [31
•], was cloned and gain-of-function
and loss-of-function experiments with this gene could help
to address this putative role. It had previously been
postu-lated that a stylar AGP may act as a pollen attractant, but
this idea has since been questioned. The role of the stylar
protein itself could now be re-examined by using
molecu-lar genetics [32].
Wall differentation
When a cell has chosen from its range of potential fates, it
differentiates. Expansin is thought to loosen noncovalent
associations between wall polysaccharides, allowing turgor
pressure to drive wall creep. Four expansin genes
(
OsEXP1
–
OsEXP4
) were shown to be expressed in
elon-gating rice tissues [33
••], and an antiserum to a cucumber
homolog of one of them was used to show that the protein
itself occurs at sites of elongation [34
•]. Immunoblotting
also revealed that submerged and elongating internodes
contained more expansin than internodes grown in air
[35
•], linking expansin accumulation in the wall to cell
and tissue elongation. Interestingly, expansin-coated
beads applied to tomato shoot apices generated small
bulges, some of which developed into leaf-like structures
bearing trichomes and expressing the gene encoding the
small subunit of ribulose bisphosphate carboxylase
rbcS
[36
••]. These ectopic structures reversed the phyllotaxy
[36
••], suggesting that expansin, by generating wall
relax-ation, can have direct and profound effects on
plant morphogenesis.
The tomato expansin Le-EXP1 was found to be regulated
by ethylene, and cDNAs related by sequence homology to
[image:3.612.57.565.93.370.2]Le-EXP1
were shown to be upregulated during
ethylene-induced ripening in melon and strawberry [37
•]. Ethylene
may, therefore, influence plant morphogenesis in part by
controlling expansin activity. Grass pollen group I allergens
were also found to be expansins, likely facilitating pollen
penetration of the stigma [38
•]. Thus, expansins are
regu-lated during development and by environmental factors,
and play diverse role in plant growth.
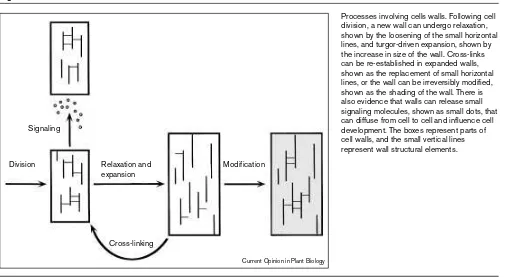
Figure 2
Processes involving cells walls. Following cell division, a new wall can undergo relaxation, shown by the loosening of the small horizontal lines, and turgor-driven expansion, shown by the increase in size of the wall. Cross-links can be re-established in expanded walls, shown as the replacement of small horizontal lines, or the wall can be irreversibly modified, shown as the shading of the wall. There is also evidence that walls can release small signaling molecules, shown as small dots, that can diffuse from cell to cell and influence cell development. The boxes represent parts of cell walls, and the small vertical lines represent wall structural elements. Signaling
Division Modification
Cross-linking Relaxation and expansion
Some HRGPs [39] are also thought to condition cell
expansion. The tomato extensins encoded by
DIF
10 and
DIF
54 are preferentially expressed in root hairs, and
inhi-bition of tomato prolyl hydroxylation with
3,4-dehydro-L-proline caused the appearance of short
root hairs [40
•]. Also, the soybean SbHRGP3 extensin
was found to accumulate in maturing roots at a time when
root hairs are growing [41
•]. These experiments link
extensins with cell elongation, and it would be
interest-ing to see if the
Arabidopsis
mutant
cow
1, which correctly
initiates root hairs but fails to allow root hair elongation
[42
••], is affected in an extensin gene. Interestingly,
treat-ment of
Arabidopsis
seedlings with
β
-glucosyl Yariv
reagent resulted in bulging epidermal cells similar to the
epidermal cells of AGP-deficient
reb
1 mutants [43
•],
sug-gesting that different kinds of HRGPs could have
separate but related roles in cell enlargement.
Xyloglucan endotransglycosylases are believed to allow
slippage in the wall by cutting adjacent xyloglucan chains
and rejoining the chains with one another [44].
Recombinant TOUCH 4 (TCH4) uses xyloglucan
poly-mers as donor and acceptor molecules in the
transglycosylation reaction it catalyses [45
••]. The fucosyl
content of either the donor or acceptor xyloglucan affects
the rate of the reaction [45
••], suggesting that control of
xyloglucan fucosylation is part of an endogenous
mecha-nism for regulating changes in the mechanical properties
of a wall. Also, tomato xyloglucan endotransglycosylase
(
LeEXT
) expression was found in elongating regions of
tomato hypocotyls [46
•], and antisera to the TCH4
revealed accumulation in expanding cells in
Arabidopsis
leaf bases, hypocotyls, and vascular tissues [47
•]. Thus,
these studies support the view that xyloglucan
endo-transglycosylases play a role in cell expansion.
Expanded cells can undergo wall re-rigidification and
modification. Oxidative cross-linking of tyrosine
residues in wall proteins, which is also brought about by
H
2O
2, is thought to be one such rigidification
mecha-nism. An important early step in this reaction is the
generation of di-isodityrosine, and pulcherosine (a trimer
of tyrosine and isodityrosine residues coupled by a
biphenyl linkage) was identified in a wall hydrosylate of
tomato [48
••]. Steric considerations suggested that only
inter-polypeptide cross-links and/or wide
intrapolypep-tide loops are tolerated in the oxidative reaction
involving tyrosine and isodityrosine, suggesting that
pul-cherosine bridges wall proteins and acts as an
intermediate in wall cross-linking. Levels of H
2O
2in
plants are regulated by control over oxidases and
cata-lases, so it may be that oxidative cross-linking is subject
to developmental control.
An antiserum localized a glycine-rich protein in only the
primary walls of the xylem tracheary elements,
demon-strating that the walls of these cells are actively modified
rather than degraded [49
••]. These proteins probably
provide the xylem cell walls with special physical
prop-erties. The disposition of 1,4-
β
-D-galactose residues in
tomato fruit pectin was localized with an antiserum and
a Mab. These were present in the walls other than
epi-dermal and sub-epiepi-dermal walls [50
•]. 1,4-
β
-D-galactans
are known to be present in some of the most flexible wall
polysaccharides, so the absence of these galactans from
the epidermal cell walls may also contribute to the
cross-linking of these walls. Modification of wall protein and
polysaccharide may, therefore, occur in concert during
wall specification.
Conclusions
Numerous reports over the past year have thrown new
light on the fundamental role of the cell wall in plant
mor-phogenesis. Structural glycoproteins are involved with the
division of a cell, and structural and catalytic proteins are
necessary for expansion during cell differentiation.
Polysaccharides are among the targets for these proteins
and modifications of polysaccharides are involved with
wall loosing, rigidification, and modification. Cell–cell
interactions can be defined by cell walls, and soluble
sig-nals probably released from walls can control cell
metabolism and development.
These advances offer important new insight into the
func-tions of cell walls, and it is with function that research into
walls should remain. Additional and refined genetic
screens for
Arabidopsis
plants defective in wall
carbohy-drate or in the perception of a signal released from a wall
will be invaluable in this respect. Forward and reverse
genetic approaches utilizing genes coding for structural
proteins and enzymes involved with wall biosynthesis and
turnover will complement this. With thorough chemical
and immunochemical analysis of such plants, wall
struc-tures and signals can be linked to functions.
Acknowledgements
I thank The Royal Society of London for a University Research Fellowship.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
•
of special interest••
of outstanding interest1. Brownlee C, Berger F: Extracellular matrix and pattern in plant embryos: On the lookout for developmental information.Trends Genet1995, 11:344-348.
2. Cosgrove DJ: Assembly and enlargement of the primary cell wall
• in plants. Ann Rev Cell Biol1997, 13:171-120.
Control of cell expansion is essential for cell and plant morphogenesis. The different ways that a wall can become loosened are discussed in this article. There is detailed coverage of the role of expansin proteins in wall loosening.
3. Doerner P: Radicle development(s).Curr Biol1995, 5:110-112. 4. Dolan L, Linstead P, Roberts K: Developmental regulation of pectic
• polysaccharides in the root meristem of Arabidopsis.J Exp Bot 1997, 48:713-720.
5. Ito M, Kodama H, Komamine A, Watanabe A: Expression of extensin
• genes is dependent on the stage of the cell cycle and cell proliferation in suspension-cultured Catharanthus roseuscells.
Plant Mol Biol1998, 36:343-351.
The expression of two extensin genes is shown to occur during G2 and S phases of the cell cycle. It would be interesting if a feedback control, from wall to nucleus, were operating to drive the cycle in plants.
6. Langhan KJ, Nothnagel EA: Cell surface arabinogalactan-proteins
• and their relation to cell viability and proliferation.Protoplasma 1997, 196:87-98.
The authors demonstrate a relationship between the AGP composition of the cell wall and responses to staining with Yariv reagent. Complexing AGPs with Yariv reagent blocks cell division, and the reversibiity of this block depends on the composition of the AGP. It may turn out that HRGPs play an important role in cell cycle progression.
7. Gaffe J, Tiznado ME, Handa AK: Characterization and functional
• expression of a ubiquitously expressed tomato pectin methylesterase.Plant Physiol1997, 114:1547-1556.
Pectin methylesterification is known to be developmentally regulated, and the level of esterification can reveal nondividing cells and the incipient anato-my of a root. Experiments with this gene could alter the patterns of pectin esterification, and thereby enable questions about the role of the esterifica-tion in plant development.
8. Woo HH, Hawes MC: Cloning of genes whose expression is
• correlated with mitosis and localized in dividing cells in root caps of Pisum sativumL.Plant Mol Biol1997, 35:1045-1051.
This paper reports a gene encoding an extensin that is expressed in non-dividing root cells. Related genes may be expressed at certain stages of the cell cycle, and overall there may be a role for extensins in cell division. Manipulation of such genes will be interesting.
9. Delmer D, Amor Y: Cellulose biosynthesis.Plant Cell1995,
7:987-1000.
10. Baskin TI, Berzner AA, Hoggart R, Cork A, Williamson RE: Root morphology mutants in Arabidopsis thaliana.Aust J Plant Physiol 1992, 19:427-437.
11. Arioli T, Peng L, Betzner AS, Burn J, Wittke W, Herth W, Camilleri C, •• Höfte H, Plazinski J, Birch R, Cork A, Glover J, Redmond J, Williamson
RE: Molecular analysis of cellulose synthesis in Arabidopsis.
Science1998, 279:717-720.
Chemical and structural analysis and map-based cloning identified RSW1 as the gene encoding the catalytic subunit of cellulose synthase. This paper represents the latest in a series of advances in the molecular biology of cel-lulose synthesis, and also elegantly demonstrates that the correct deposition of cellulose is essential for cell and plant morphogenesis.
12. Turner SR, Somerville CR: Collapsed xylem phenotype of
•• Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall.Plant Cell1997, 9:689-701.
A normal pattern of cellulose deposition may be necessary for lignin deposi-tion and wall reinforcement. This has important implicadeposi-tions for wall assembly and development, but also shows that it is possible to screen EMS populations by using anatomical methods, and to recover interesting and important mutants in this way.
13. Brummel DA, Catala C, Lashbrook CC, Bennett AB: A
membrane-• anchored E-type endo-1,4-b-glucanase is localized on Golgi and plasma membranes of higher plants. Proc Natl Acad Sci USA 1997, 94:4794-4799.
Glucanases are interesting proteins with numerous potential functions in plant development and defense. The function of this one is not known, but a role in cellulose synthesis would be interesting and perhaps feed into a growing picture of the mechanism of cellulose metabolism.
14. Faik A, Chilsehe C, Sterling J, Maclachlan G: Xyloglucan
galactosyl-• and fucosyltransferase activities from pea epicotyl microsomes.
Plant Physiol1997, 114:245-254.
This paper advances our understanding of xyloglucan biosynthesis by show-ing that the side-chain galactose is attached at the xyloglucose next the the xyloglucose at the reducing end of xyloglucan. Detailed studies on polysac-charide biosynthesis may reveal how wall compounds such as xyloglucan conditions stage-specific effects in plant development.
15. Doong RL, Mohnen D: Solubilization and charaterization of a
• galacturonosyltransferase that synthesises the pectic polysaccharide homoglacturonan.Plant J1998, 13:363-374. The characterization of the glycosyltransferase that synthesizes homo-galacturonan should aid analysis of homohomo-galacturonan biosynthesis, and may help to reveal how this wall pectin conditions stage-specific effects in morphogenesis.
16. Robertson D, Mitchell GP, Gilroy JS, Gerrish C, Bolwell GP, Slabas • AR: Differential extraction and protein sequencing reveals major differences in patterns of primary cell wall proteins from plants.
J Biol Chem1997, 272:15841-15848.
The authors describe clear species-differences in extractable cell wall pro-teins, some of which are novel proteins. These species used in this study were not closely related, and it would be interesting to perform similar com-parisons on genera from a single family, or species from a single genus.
17. Reiter WD, Chapple C, Somerville CR: Mutants of Arabidopsis
•• thalianawith altered cell wall polysaccharide composition.Plant J 1997, 12:335-345.
The identification of 11 mutants in which the levels of specific monosaccha-rides are reduced shows that a biochemical screening procedure can identify mutants altered in different kinds of cell wall polysaccharides and/or glycoproteins. As some of the mutants were dwarfs, cloning, sequencing, and manipulating the affected genes may be useful in relating wall composi-tion and funccomposi-tion.
18. van den Berg C, Willemsen V, Hendriks G, Weisbeek P, Scheres B: •• Short-range control of cell differentiation in the Arabidopsisroot
meristem.Nature1997, 390:287-289.
Laser ablation and genetic studies indicate that short-range signals originat-ing in the quiescent center inhibit the differentiation of surroundoriginat-ing meristem cells. The signals are not known; one possibility is that they originate in cell walls. A great paper.
19. Lolle SJ, Berlyn GP, Engstrom EM, Krolikowski KA, Reiter WD, Pruitt •• RE: Developmental regulation of cell interactions in the
Arabidopsis fiddlehead-1mutant: a role for the epidermal cell wall and cuticle.Dev Biol1997, 189:311-321.
One possibily arising from fdh1 is that cell walls serve as molecular filters, and refine the profile of the signaling molecules that move from one cell to another during patterning and cell differentiation. It would be interest-ing to see if fdh1walls contained higher than nomal levels of the any of the lipids that promote pollen germination on stigmaless Petuniastyles. A fascinating paper.
20. Wolters-Arts M, Lush WM, Mariani C: Lipids are required for
•• directional pollen-tube growth.Nature1998, 392:818-821. This demonstration that trilinolein, an unsaturated fatty acid present in the lipid fraction of Petuniastigmatic exudate, allows pollen to penetrate stig-maless pistils is a novel and interesting discovery, positioning fatty acids as extracellular signaling compounds. This report deals with the interaction between stigma and pollen; it will be intersting to see if other lipids are involved with other cell–cell interactions in the vegetative parts of plants.
21. Mollard A, Domon JM, David H, Joseleau JP: Xylose-rich
polysaccharides from the primary walls of embryogenic cell lines of Pinus caribaea.Int J Biol Macromol1997, 21:189-194. 22. McCabe PF, Valentine TA, Forsberg LS, Pennell RI: Soluble signals
•• from cells identified at the cell wall establish a developmental pathway in carrot.Plant Cell1997, 9:2225-2241.
Cell sorting with Mabs was used to show that cells identifiable at their walls control the progression of other cells into somatic embryos. Wall structures may also identify interacting cells in whole plants. A molecular analysis of these wall-derived cells could turn up some interesting and useful genes.
23. Roberts AW, Donovan SG, Haigler CH: A secreted factor induces
• cell expansion and formation of metaxylem-like tracheary elements in xylogenic suspension cultures of Zinnia.Plant Physiol 1997, 115:683-692.
The transdifferentiation of Zinniamesophyll cells in tracheary elements may be useful in understanding cell communication and committment. This paper reports a soluble factor, probably an oligosaccahride, that is relased from such cells and that affects several aspects of cell develop-ment. It will be interesting to obtain the strucutre of this factor, and to test its specific activities.
24. Dinand E, Excoffier G, Liénart Y, Vignon MR: Two
• rhamnogalacturonide tetrasaccharides isolated from semi-retted flax fibers are signaling molecules in Rubus fruticosusL. cells.
Plant Physiol1997, 115:793-801.
A water extraction yields a mixture of pectic oligosaccharides including two rhamnogalacturonide tetrasaccharides able to activate D-glycohydrolases in Rubus fructicosuscells. This shows that the tetrasaccharides are likely to diffuse from cell wall in vivo, and may therefore represent signal molecules with natural functions.
25. Knox JP: The use of antibodies to study the architecture and
• developmental regulation of plant cell walls.Int Rev Cytol1997,
171:19-120.
26. Casero PJ, Casimiro I, Knox JP: Occurrence of cell surface
• arabinogalactan-protein and extensin epitopes in relation to pericycle and vascular tissue development in the root apex of four species.Planta1998, 204:252-259.
That the expression of HRGP epitopes in the pericycles of pea, radish, car-rot and onion reflects the pattern of vascular development suggests that the HRGPs are involved with this patterning. What is needed is a functional analysis of the significance of these epitope expression patterns.
27. Kjellbom P, Snogerup L, Stöhr C, Reuzeau C, McCabe PF, Pennell RI: • Oxidative cross-linking of plasma membrane arabinogalactan
proteins.Plant J1997, 12:1189-1196.
This paper adds AGPs to the list of cell wall molecules that can be cross-linked by H2O2. The significance is not clear, but the finding that certain epitopes that are developmentally regulated in roots occur only on cross-linked AGPs suggests that H2O2may play a role in pattern formation.
28. Thompson HJM, Knox JP: Stage-specific responses of
• embryogenic carrot cell suspension cultures to arabinogalactan protein-binding b-glucosyl Yariv reagent.Planta1998, 205:32-38. The addition of β-glucosyl Yariv reagent to an embryo culture can cause the appearance of shoot-less embryos, suggesting that the AGPs at the future shoot pole play a role in shoot morphogenesis. Difficult to interpret, but an interesting paper and potentially important.
29. Ding L, Zhu JK: A role for arabinogalactan-proteins in root
• epidermal cell expansion. Planta1997, 203:289-294.
There is considerable evidence that HRGPs such as extensins play an important role in cell expansion. This paper suggests that wall AGPs also help to condition the physical properties of epidermal cell walls. Exactly how they might do this is not known, but this and other studies do argue in favor of this idea.
30. Triplett BA, Timpa JD: b-glucosyl and a-galactosyl Yariv reagents
• bind to cellulose and other glucans.J Agric Food Chem1997,
45:4650-4654.
The authors show β-glucosyl Yariv reagent is not specific for AGPs, and can bind to other cell wall polysaccharides in certain circumstances. The important point from this paper is that studies involving Yariv reagent should always feature controls with the α-glucosyl Yariv reagent or Mabs to AGPs.
31. Schultz CJ, Hauser K, Lind JL, Atkinson AH, Pu ZY, Anderson MA, • Clarke AE: Molecular characterization of a cDNA sequence
encoding the backbone of a style-specific 120kDA glycoprotein which has features of both extensins and arbinogalactan proteins.
Plant Mol Biol 1997, 35:833-845.
The core protein encoded by NaPRP5 is soluble and expressed in styles. The encoded protein contains three domains, one of which (at the carboxy-terminal) is similar to a part of a galactose-rich style glycoprotein. The sharing of different domains between distinct HRGPs shows further complexity in this class of wall molecules.
32. Sommer-Knudsen J, Lush WM, Bacic, A, Clarke AE: Re-evaluation of the role of a transmitting tract-specific glycoprotein on pollen tube growth.Plant J1998, 13:529-535.
33. Cho H-T, Kende H: Expression of expansin genes is correlated
•• with growth in deepwater rice.Plant Cell1997, 9:1661-1671. Pleasing demonstration at the RNA level that expression of three rice expansin genes occurs in the elongating parts of the coleoptile, root, leaf and internode of deepwater rice, and also correlates with acid-induced wall extensibility in vitro. An elegant and convincing correlation between expansin and elongation.
34. Cho H-T, Kende H: Expansins in deepwater rice internodes.Plant • Physiol1997, 113:1137-1143.
The authors demonstrate at the protein level that two expansins occur in the walls of rice internodes, and that they may mediate acid-induced wall extensibility. Clear correlative evidence for a role for expansin in intern-ode elongation.
35. Cho H-T, Kende H: Expansins and internodal growth of deepwater
• rice.Plant Physiol1997, 113:1145-1151.
An antiserum raised to a cucumber expansin was used as a probe to show that rice expansin occurs primarily in the internode elongation zone. This study links expansin accumulation to extension of the internode, and goes on to localize these expansins to the walls of the vascular bundles and the inner epidermal cells surrounding the internodal cavity.
36. Fleming AJ, McQueen-Mason S, Mandel T, Kuhlemeier C: Induction
•• of leaf primordia by the cell wall protein expansin.Science1997,
276:1415-1418.
Beads loaded with expansin induce leaf-like primordia on the shoot apex of tomato, and can reverse the direction of the phyllotaxy. These data suggest a scheme for plant morphogenesis that is broader than the cause-and-effect view of signal, signal transduction, and gene activation in the control plant form. A fascinating paper.
37. Rose JKC, Lee HH, Bennett AB: Expression of a divergent
• expansin gene is fruit-specific and ripening regulated.Proc Natl Acad Sci USA1997, 27:5955-5960.
It is shown that tomato expansin is specifically expressed in ripening fruits, suggesting that it has a role in the ripening process. Evidence for expansin function in cell elongation, pollen penetration and here ripening suggests that expansin proteins play numerous and important roles in development, morphogenesis and reproduction.
38. Cosgrove DJ, Bedinger P, Durachko DM: Group I allergens of grass
• pollen as cell wall-loosening agents.Proc Natl Acad Sci USA 1997, 94:6559-6564.
The presence of expansins in the walls of wheat pollen grains suggest that pollen expansins might act on the walls of the stigma and style to aid pene-tration. It is also shown that pollen expansins can be used to identify mRNAs coding for expansins in the vegetative parts of other species. Pollen could be used as a rich source of expansins.
39. Kieliszewski MJ, Lamport DTA: Extensin: Repetitive motifs, functional sites, post-tranlational codes, and phylogeny.Plant J 1994, 5:157-172.
40. Bucher M, Schroeer B, Willmitzer L, Riesmeier JW: Two genes
• encoding extensin-like proteins are predominantly expressed in tomato root hair cells.Plant Mol Biol1997, 35:497-508.
The identification of two extensin genes which are preferentially expressed in root hair cells, and the blockage of root hair growth by the prolyl hydroxylse inhibitor 3,4-dehydro-L-proline, suggests a function for extensins in root hair development. A clear and well-performed study.
41. Hoon AJ, Choi K, Kim SG, Myung KY, Do CY, Seob LJ: Expression of
• a soybean hydroxyproline-rich glycoprotein gene is correlated with maturation of roots. Plant Physiol1998, 116:671-679. The expression of SbHRGP3 increases during root development, showing that expression is developementally-regulated. The function of the HRGP is not known, but one possibility is that it is involved with modifying the expan-sibility of the cell walls. The gene may be useful for studies targeted to resolving this function.
42. Grierson CS, Roberts K, Feldmann KA, Dolan L: The COW1 locus of
•• Arabidopsis acts after RHD2, and in parallel with RHD3 and TIP1, to determine the shape, rate of elongation, and number of root hairs produced from each site of hair formation.Plant Physiol 1997, 115:981-990.
Root hair abnormalities characteristic of COW1are consistent with a defect in an extensin gene. It would be interesting to see if these plants fail to express or misexpress any of the extensins that are known to occur in Arabidopsis. Conceivably, overexpression of such genes might make longer root hairs.
43. Ding L, Zhu JK: A role for arabinogalactan-proteins in root
• epidermal cell expansion.Planta1997, 203:289-294.
Treatment of Arabidopsisroots with Yariv reagent causes the appearance of balloon-like swellings in the epidermal cells, thereby phenocopying the mutant reb1. Yariv reagent binds AGPs, so this paper lends weight to the view that certain AGPs play a mechanical role in cell expansion control. Further analysis of reb1 will be interesting.
44. Xu W, Purugganan MM, Polisensky DH, Antosiewic DM, Fry SC, Braam J: Arabidopsis TCH4, regulated by hormones and the environment, encodes a xyloglucan endotransglycosylase.Plant Cell1995, 7:1555-1567.
45. Purugganan MM, Braam J, Fry S: The Arabidopsis TCH4 xyloglucan
•• endotransglycosylase. Substrate specificity, pH optimum, and cold tolerance.Plant Physiol1997, 115:181-190.
Purification has allowed the authors to detail the substrate requirements of the ArabidopsisXET. A detailed understanding of this enzyme is likely to be important for a complete picture of wall loosening and responses to endoge-nous and environmental signals.
46. Catalá C, Rose JKC, Bennett AB: Auxin regulation and spatial
• localization of an endo-1,4-b-D-glucanase and a xyloglucan endotransglycosylase in expanding tomato hypocotyls. Plant J 1997, 12:417-426.
The authors show unique and overlapping expression patterns an endo-1,4-β-glucanase and an XET. These genes have the potential to promote wall loosing and disassembly during with growth. One interesting issue raised by these data is that they may act co-operatively to achieve this.
47. Antosiewicz DM, Purugganan MM, Polisensky DH, Braam J: Cellular
• localization of Arabidopsis xyloglucan endotransglycosylase-related proteins during development and after wind stimulation.
Plant Physiol1997, 115:1319-1328.
48. Brady JD, Sadler IH, Fry SC: Pulcherosine, an oxidatively coupled
•• trimer of tyrosine in plant cell walls: its role in cross-link formation.Phytochemistry1998, 47:349-353.
This paper completes a series of publications establishing the chemistry of one class of interpolypeptide cell wall cross-links thought to be modifiers of the wall. When it is known how the polypeptid or the H2O2can be regulat-ed, it will be possible to make important predictions on the role of pulcherosine in development and morphogenesis.
49. Ryser U, Schorderet M, Zhao GF, Studer D, Ruel K, Hauf G, Keller B: •• Structural cell-wall proteins in protoxylem development: evidence
for a repair process mediated by a glycine-rich protein.Plant J 1997, 12:79-111.
A proline-rich protein and a glycine-rich protein are shown to occur in dif-ferent parts of protoxylem cell walls. Along with cellulose, glycine-rich proteins such as this and pectic galactans may function as a scaffold for lignin assembly.
50. Jones L, Seymore, GB, Knox JP: Localization of pectic galactan in
• tomato cell walls using a monoclonal antibody specific to (1-4)-b -D-galactan.Plant Physiol1997, 113:1405-1412.