Analisis Sediaan Farmasi
Analisis Sediaan Farmasi
Henry K.S.,M.Si.Apt,
Henry K.S.,M.Si.Apt,
Jadwal Kuliah
Jadwal Kuliah
Minggu
Minggu Bahan kajianBahan kajian
II Validasi metode analisisValidasi metode analisis II
II Validasi metode analisisValidasi metode analisis III
III Pemisahan Pemisahan komponen aktif dari komponen aktif dari bahan pembantu sediaan bahan pembantu sediaan obat, serta pemobat, serta pemilihan metodeilihan metode
analisis analisis
IV
IV Analisis kuantitatif sediaan obat monokomponen (padat & semi padat) secaraAnalisis kuantitatif sediaan obat monokomponen (padat & semi padat) secara
spektrofotometri, spektrofluorometri dan elektrokimia spektrofotometri, spektrofluorometri dan elektrokimia
V
V Analisis kuantitatif sediaan obat monokomponen (cair dan steril) secara spektrofotometri,Analisis kuantitatif sediaan obat monokomponen (cair dan steril) secara spektrofotometri,
spektrofluorometri dan elektrokimia spektrofluorometri dan elektrokimia
VI
VI Analisis kuantitatif sediaan obat multikomponen (padat & semi padat) secaraAnalisis kuantitatif sediaan obat multikomponen (padat & semi padat) secara
spektrofotometri dan spektrofluorometri spektrofotometri dan spektrofluorometri
VII
VII Analisis kuantitatif sediaan obat multikomponen (Cair dan Steril) secara spektrofotometriAnalisis kuantitatif sediaan obat multikomponen (Cair dan Steril) secara spektrofotometri
dan spektrofluorometri dan spektrofluorometri
VIII
VIII Penentuan dan Identifikasi permasalahan dalam hal Penentuan dan Identifikasi permasalahan dalam hal analisisanalisis IX
IX Analisis kuantitatif Analisis kuantitatif sediaan obat sediaan obat multikomponen multikomponen secara KCKTsecara KCKT X
X Analisis kuantitatif sediaan Analisis kuantitatif sediaan obat multikomponen secara obat multikomponen secara KLKLT dan KGT dan KG XI
XI Analisis Sediaan KosmetikaAnalisis Sediaan Kosmetika XII
XII Analisa Sediaan Makanan dan minumanAnalisa Sediaan Makanan dan minuman XIII
XIII Analisa dalam Sampel BiologisAnalisa dalam Sampel Biologis XIV
Daftar Pustaka:
Daftar Pustaka:
•• United States PharmacopoeiaUnited States Pharmacopoeia
•• Horwitz, W., and Latimer, Horwitz, W., and Latimer, G.W., 2005, Official MethodsG.W., 2005, Official Methods of Analysis, AOAC International, Maryland
of Analysis, AOAC International, Maryland
•• Robert V Smith, et. Al, Textbook of BiopharmaceuticRobert V Smith, et. Al, Textbook of Biopharmaceutic Analysi
Analysis,1981.s,1981.
•• Cahyadi Cahyadi W. W. , , 2006,2006, Analisi Analisis dan Aspek Kess dan Aspek Kesehatanehatan Bahan Tambahan Pangan
Bahan Tambahan Pangan, Bumi Aksara: Jakarta, Bumi Aksara: Jakarta •• Rohman A., dan I.G. Gandjar, 2007,Rohman A., dan I.G. Gandjar, 2007, MetodeMetode
Kromatografi untuk Analisis Makanan
Kromatografi untuk Analisis Makanan, Pustaka Pelajar:, Pustaka Pelajar: Yogyakarta
Yogyakarta
•• Mitra S., 2003,Mitra S., 2003, Sample Preparation Techniques inSample Preparation Techniques in Analytica
Analisis Sediaan Farmasi
Analisis Sediaan Farmasi
Analisis = ??
Analisis = ?????
???
Sediaan Farmasi = ????
Sediaan Farmasi = ????
C
Validation
Proses penilaian terhadap parameter
analitik tertentu, berdasarkan percobaan
laboratorium, untuk membuktikan bahwa
parameter tersebut memenuhi syarat
The Validation Process
Validation
Method
Validation
System
Suitability
Hardware
Software
Validation (4M)
• Man
• Machine
• Material
• Method
Qualification
Qualification
•• Qualification is a subset of the validation
Qualification is a subset of the validation
process that verifies module and system
process that verifies module and system
performance prior to the instrument being
performance prior to the instrument being
placed on-line.
placed on-line.
•• If the instrument is not qualified prior to
If the instrument is not qualified prior to
use and a problem is encountered, the
use and a problem is encountered, the
source of the problem will be
source of the problem will be difficult to
difficult to
identify.
The Validation Timeline
The Validation Timeline
Vendor’s Site
Vendor’s Site
User’s
User’s
Site
Site
User’s
User’s
Site
Site
Structural
Structural
and
and
Software
Software
Qualification
Qualification
Qualification
Qualification
IQ
IQ
OQ
OQ
PQ
PQ
Calibration
Calibration
and
and
Maintenance,
Maintenance,
System
System
Suitability
Suitability
BeforeInstallation Qualification (IQ)
Installation Qualification (IQ)
•• The IQ process can be divided into two
The IQ process can be divided into two
steps: preinstallation and physical
steps: preinstallation and physical
installation.
installation.
•• During the preinstallation, all the
During the preinstallation, all the
information pertinent to the proper
information pertinent to the proper
installation, operation, and maintenance of
installation, operation, and maintenance of
the instrument is reviewed.
the instrument is reviewed.
•• Site requirements and the receipt of all of
Site requirements and the receipt of all of
the parts, pieces, manuals, etc., necessary
the parts, pieces, manuals, etc., necessary
to perform the installation are confirmed.
•• During the physical installation, serial
During the physical installation, serial
numbers are recorded, and all of the
numbers are recorded, and all of the
fluidic, electrical, and communication
fluidic, electrical, and communication
connections are made for components in
connections are made for components in
the system.
the system.
•• Documentation describing how the
Documentation describing how the
instrument was installed, who
instrument was installed, who performed
performed
the installation, and other miscellaneous
the installation, and other miscellaneous
details should be archived.
details should be archived.
Installation Qualification (IQ)
Operational Qualification (OQ)
• The OQ process ensures that the specific
modules of the system are operating
according to the defined specifications for
accuracy, linearity and precision.
• This process may be as simple as
verifying the module’s self diagnostic
routines, or it may be performed in more
depth by running specific tests, for
example, to verify detector wavelength
accuracy, flow rate, or injector precision.
Performance Qualification (PQ)
• The PQ process verifies system performance.
• PQ testing is conducted under actual running
conditions across the anticipated working range.
• In practice, however, OQ and PQ are frequently
performed together, particularly for linearity and
precision (repeatability) tests, which can be
conducted more easily at the system level.
• For HPLC, the PQ test should use a method with
a well-characterized analyte mixture, column,
and mobile phase. A system suitability must be
performed.
• Proper documentation supporting the PQ
process should be archived.
Validation
• Process Validation
– Prospective Validation – Ongoing Validation
– Re-Validation: After change, Periodic, – Retrospective Validation
• Analytical Method Validation
– Specificity, Linearity, Precision, Accuracy/Recovery, Ruggedness
• Cleaning Validation
• Utility System Validation • Computer Validation
What is not Analytical Method Validation?
• Calibration
The Process of Performing Tests on Individual System Components to Ensure Proper function
• System Suitability
Test to verify the proper functioning of the operating system, i.e., the electronics, the equipment, the
HPLC Detector calibration
• Wavelength Accuracy
• Linear Range
• Noise Level
• Drift
Typical System Suitability Test
• Minimum Resolution of 3.0 between
the analyte peak and internal
standard peaks
• Relative Standard Deviation of
replicate standard injections of not
more than 2.0%
Method Life Cycle
Validation
Verification vs. Validation
• Compendial vs. Non-compendial Methods
– Compendial methods-Verification – Non-compendial methods-Validation
Today’s Validation Requirements
ICH/USP
GMPs
GMP Validation Parameters
• Accuracy
• Specificity
• Sensitivity
FDA Validation Parameters
• Accuracy
• Precision
• Linearity
(& Range)
• Specificity
(& Determination Limit)
• Recovery
• Ruggedness
ICH/USP Validation Requirements &
Parameters
• Specificity • Linearity • Range • Accuracy • Precision – Repeatability – Intermediate Precision – Reproducibility • Limit of DetectionICH
I nter national Conf er ence on H armoni zation
Specificity Linearity and Range Accuracy Precision Limit of Detection Limit of Quantitation Ruggedness Robustness
USP
USP Data Elements Required For Assay
Validation
* May be required, depending on the nature of the specific test.
Analytical Performance Parameter Assay Category 1 Assay Category 2 Assay Category 3 Quantitative Limit Tests
Accuracy Yes Yes *
Precision Yes Yes No Yes Specificity Yes Yes Yes
LOD No No Yes
LOQ No Yes No
Linearity Yes Yes No *
Range Yes Yes * *
USP Categories
• Category 1: Quantitation of major components or active ingredients
• Category 2: Determination of impurities or degradation products
• Category 3: Determination of performance characteristics
ICH Validation Characteristics vs. Type of
Analytical Procedure
Type of Analytical Procedure Identification Impurity testing Assay Quantitative Limit TestsAccuracy No Yes No Yes Precision
Repeatability No Yes No Yes Interm. Prec. No Yes No Yes Specificity Yes Yes Yes Yes
LOD No No Yes No
LOQ No Yes No No
Linearity No Yes No Yes Range No Yes No Yes
Method Validation for USP
• Method validation, according to the United
States Pharmacopeia (USP), is performed to
ensure that an analytical methodology is
accurate, specific, reproducible, and rugged
over the specified range that an analyte will be
analyzed.
• Method validation provides an assurance of
reliability during normal use and is sometime
described as the proces of providing
documented evidence that the method does
what it is intended to do.
KAPAN DILAKUKAN?
1. Pengembangan metode analisis (MA) yang
telah ada misalnya untuk:
- Matriks sampel yang spesifik
- Memperbaiki “Analytical Performance” MA
dengan adanya instrument atau teknik baru
- MA yang terlalu mahal, memakan banyak
waktu & energi
2. Terhadap MA yang dibuat dari
modifikasi metode resmi (standard
yang telah dipublikasi secara
internasional, regional atau nasional;
jurnal ilmiah yang relevan)
TUJUAN
1. Hasil analisis absah/valid, dapat
dipercaya dan dapat dipertanggung
jawabkan secara ilmiah
2. Hasil analisis dapat menunjukkan
Accuracy vs precision
What you
would like
Accuracy vs precision
•
Poor accuracy
•
Good precision
Accuracy vs precision
•
Poor precision
•
Good accuracy
Accuracy vs precision
•
Totally hopeless!
•Poor precision
•
Poor accuracy
What would you
call this?
So what definitions do these
concepts lead us to in the
ACCURACY (1)
• The accuracy of an analytical
procedure expresses the closeness of
agreement between the value which is
accepted either as a conventional true
value or an accepted reference value
and the value found. This is
ACCURACY (2)
Assay of Drug Substance:
a) application of an analytical procedure to an
analyte of known purity (e.g. reference
material);
b) comparison of the results of the proposed
analytical procedure with those of a second
well-characterized procedure, the accuracy
of which is stated and/or defined
(independent procedure)
c) accuracy may be inferred once precision,
linearity and specificity have been
ACCURACY (3)
Assay of Drug Product:
a) application of the analytical procedure to synthetic mixtures of the drug product components to which known quantities of the drug substance to be
analysed have been added;
b) in cases where it is impossible to obtain samples of all drug product components, it may be acceptable either to:
– add known quantities of the analyte to the drug product or – to compare the results obtained from a second, well
characterized procedure, the accuracy of which is stated and/or defined (independent procedure)
c) accuracy may be inferred once precision, linearity and specificity have been established.
ACCURACY (4)
Impurities (Quantitation):
• Accuracy should be assessed on samples (drug substance/drug product) spiked with known
amounts of impurities.
• In cases where it is impossible to obtain samples of certain impurities and/or degradation products, it is considered acceptable to compare results obtained by an independent procedure.
• It should be clear how the individual or total
impurities are to be determined e.g., weight/weight
or area percent, in all cases with respect to the major analyte.
The Matrix Effect
• The matrix effect problem occurs when the unknown sample
contains many impurities.
• If impurities present in the unknown interact with the analyte to change the instrumental response or
themselves produce an
instrumental response, then a calibration curve based on pure analyte samples will give an
Analytical Method Development
• Accuracy: Application of the method to synthetic mixtures of the drug product components to which known quantities of the
analyte have been added • Recovery reduced
by ~10 – 15%
From: Analytical Method Validation and Instrument Performance Verification, Edited by Chung Chow Chan,Herman Lam, Y.C. Lee and Xue-Ming Zhang, ISBN 0-471-25953-5, Wiley & Sons
Recommended Data
• Accuracy should be assessed using a
min.
of
9 determinations over a min. of 3
concentration levels
covering the specified
range (e.g. 3 concentrations/3 replicates
each of the total analytical procedure).
• Accuracy should be reported as:
–
% recovery
by the assay of known addedamount of analyte in the sample or as
– the difference between the mean and the accepted true value together with the
Example:
• Taken from:
ASEAN Operational Manual for
Implementation of GMP ed. 2000 p.405
• Nine solutions containing different
concentrations of ketotifen fumarate
reference standard added to ketotifen
tablet batch no. 2506VAMG were
Example (continued):
Conc. of ketotifen fumarate Areadetected Recovery (%) Acceptance Criteria mg/ml % 0.280 0.320 0.360 0.380 0.400 0.420 0.440 0.480 0.520 70 80 90 95 100 105 110 120 130 1473566 1677013 1904848 1905862 2091215 2180374 2293647 2518976 2670144 99.32 99.48 100.94 100.51 100.06 100.03 100.07 101.01 98.99 Mean (recovery) : 100.04 Standard deviation : 0.699 Relative standard deviation (RSD) : 0.699 %
98.0 – 102.0 %
Accuracy
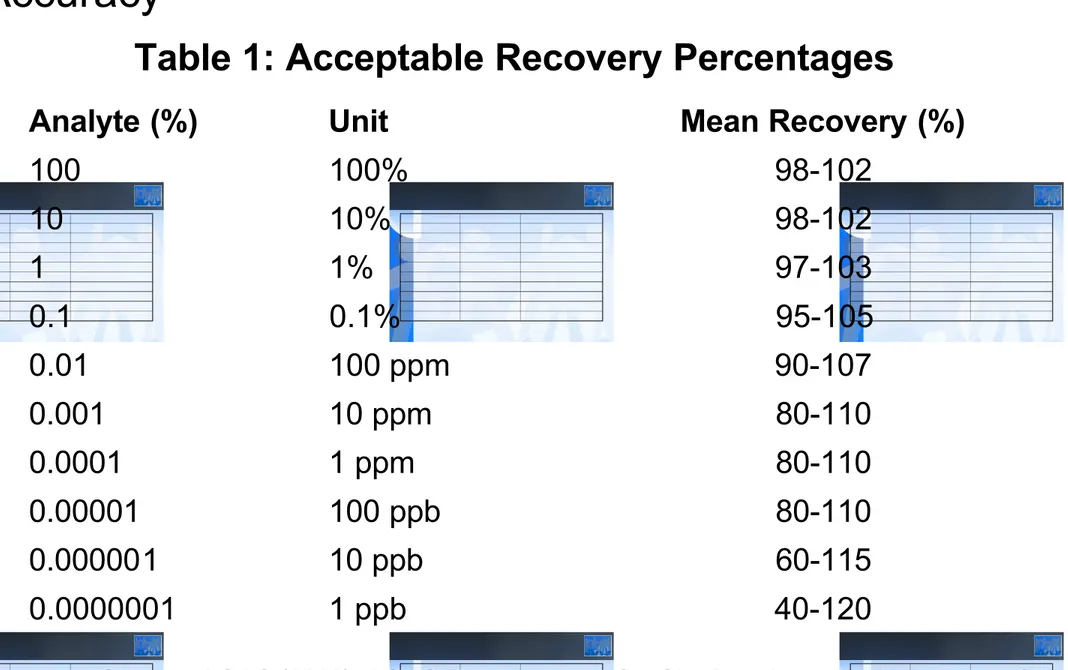
Analyte (%) Unit Mean Recovery (%)
100 100% 98-102 10 10% 98-102 1 1% 97-103 0.1 0.1% 95-105 0.01 100 ppm 90-107 0.001 10 ppm 80-110 0.0001 1 ppm 80-110 0.00001 100 ppb 80-110 0.000001 10 ppb 60-115 0.0000001 1 ppb 40-120
Table 1: Acceptable Recovery Percentages
Source: AOAC (2002). AOAC Requirements for Single Laboratory Validation of Chemical Methods. DRAFT 2002-11-07, \AOACI\eCam\Single-Lab_Validation_47.doc. http://www.aoac.org/Ag_Materials/additives/aoac_slv.pdf
PRECISION
• The precision of an analytical procedure expresses the closeness of agreement (degree of scatter)
between a series of measurements obtained from
multiple sampling of the same homogeneous sample under the prescribed conditions.
• Precision may be considered at three levels: –
repeatability,
–
intermediate precision and
–reproducibility
.• Precision should be investigated using
homogeneous, authentic samples. However, if it is not possible to obtain a homogeneous sample it may be investigated using artificially prepared samples or a sample solution.
• The precision of an analytical procedure is usually expressed as the variance,
standard deviation or
Repeatability (1)
• Repeatability expresses the precision
under the same operating conditions
over a short interval of time.
• Repeatability is also termed intra-assay
precision.
Repeatability (2)
• Repeatability should be assessed
using:
a) a minimum of 9 determinations
covering the specified range for the
procedure (e.g. 3 concentrations/3
replicates each) or
b) a minimum of 6 determinations at
100% of the test concentration.
Intermediate precision
• Intermediate precision expresses within-laboratories variations:
different days
,different analysts
,different equipment
, etc.• The extent to which intermediate precision should be established depends on the circumstances under
which the procedure is intended to be used.
• The applicant should establish the effects of random events on the precision of the analytical procedure. • Typical variations to be studied include days,
analysts, equipment, etc. It is not considered
necessary to study these effects individually. The use of an experimental design (matrix) is
Reproducibility
• Reproducibility is assessed by means
of an inter-laboratory trial.
• Reproducibility should be considered
in case of the standardization of an
analytical procedure, for instance, for
inclusion of procedures in
Recommended Data
• The
standard deviation
,
relative
standard deviation
(coefficient of
variation) and confidence interval
should be reported for each type of
precision investigated.
Example
• Taken from:
ASEAN Operational Manual for
Implementation of GMP ed. 2000 p.403
• The active ingredient, ketotifen
fumarate,
in tablets (batch no. 2506VAMG) was
assayed seven times using HPLC and
the reference standard
Example (continued)
Sample no. Concentration (mg/ml) Area detected 1 2 3 4 5 6 7 0.4 0.4 0.4 0.4 0.4 0.4 0.4 1902803 1928083 1911457 1915897 1913312 1897702 1907019 Mean : 1910896 Standard deviation : 9841.78 Relative standard deviation (RSD) : 0.515 %
Acceptance criteria: Relative standard deviation (RSD): not more than 2 %
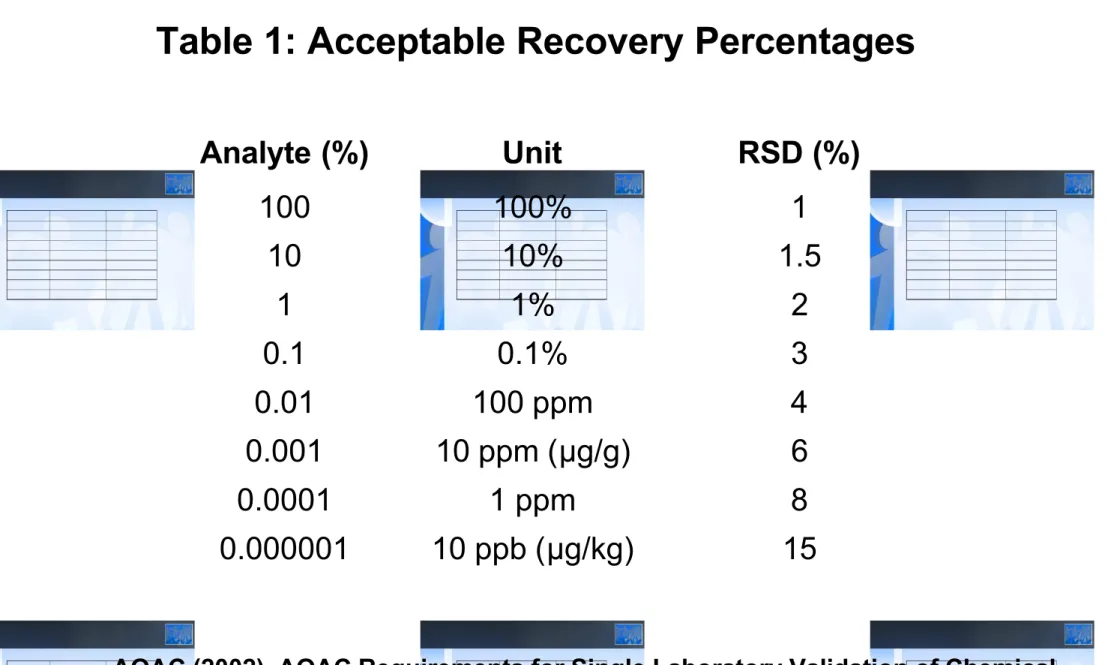
Kriteria
Secara umum:
- RSD < 1.0 % (Bahan baku obat)
- RSD < 2.0 % (Sediaan obat)
Analyte (%) Unit RSD (%) 100 100% 1 10 10% 1.5 1 1% 2 0.1 0.1% 3 0.01 100 ppm 4 0.001 10 ppm (μg/g) 6 0.0001 1 ppm 8 0.000001 10 ppb (μg/kg) 15
Table 1: Acceptable Recovery Percentages
AOAC (2002). AOAC Requirements for Single Laboratory Validation of Chemical Methods. DRAFT 2002-11-07, \AOACI\eCam\Single-Lab_Validation_47.doc. http://www.aoac.org/Ag_Materials/additives/aoac_slv.pdf.
Specificity/Selectivity
• Ability of an analytical method to measure the analyte free from interference due to other components.
• Selectivity describes the ability of an analytical method to differentiate various substances in a sample
Specificity: Impurities Assay
• Chromatographic Methods
– Demonstrate Resolution
• Impurities/Degradants Available
– Spike with impurities/degradants
– Show resolution and a lack of interference
• Impurities/Degradants Not Available
– Stress Samples
– For assay, Stressed and Unstressed Samples should be compared.
Forced Degradation Studies
• Temperature (50-60 • Humidity (70-80%)
• Acid Hydrolysis (0.1 N HCl) • Base Hydrolysis (0.1 N NaOH) • Oxidation (3-30%)
• Light (UV/Vis)
Intent is to create 10 to 30 % Degradation
Examples of pure and impure HPLC peaks
Source: LabCompliance (2007). Validation of Analytical Methods and Procedures: Tutorial. http://www.labcompliance.com/tutorial/methods/default.aspx?sm=d_d
Linearity
• Ability of an assay
to elicit a direct and
proportional
response to
changes in analyte
concentration.
Linearity Should be Evaluated
• By Visual Inspection of plot of signals vs. analyte concentration
• By Appropriate statistical methods
– Linear Regression (y = mx + b)
– Correlation Coefficient, y-intercept (b), slope (m)
• Acceptance criteria: Linear regression r 2 > 0.99
Cara penetapan
• Ditetapkan terhadap minimum
konsentrasi pada rentang minimum 50 %
- 150 % dari kadar analit
• Dihitung regresi liniernya dan didapat
persamaan regresi: Y = a + bx
RANGE
• The specified range is normally derived
from linearity studies and depends on the
intended application of the procedure.
• It is established by confirming that the
analytical procedure provides an
acceptable degree of linearity, accuracy
and precision when applied to samples
containing amounts of analyte within or at
the extremes of the specified range of the
analytical procedure.
Minimum Specified Ranges
• for the
assay
of a drug substance or a finished
(drug) product: normally from 80 - 120 % of the
test concentration
• for
content uniformity
, covering a minimum of
70 - 130 % of the test concentration
• for
dissolution
testing:
+/-20 % over the
specified range
; e.g., if the specifications for a
controlled released product cover a region
from 20%, after 1 hour, up to 90%, after 24
hours, the validated range would be 0-110% of
the label claim
Detection limit vs Quantitation
limit
‘Know that it’s there’
vs
Detection limit
(means)
Is any of it present?
Quantitation limit
How much of it is present???
Method Validation- LOD and LOQ
Sensitivity
• Limit of detection (LOD) – “the lowest content that can be measured with reasonable statistical certainty.”
• Limit of quantitative measurement (LOQ) – “the lowest concentration of an analyte that can be determined with acceptable precision (repeatability) and accuracy under
the stated conditions of the test.”
1. Based in Visual Evaluations
- Used for non-instrumental methods
2. Based on Signal-to Noise-Ratio
- 3:1 for Detection Limit
- 10:1 for Quantitation Limit
3. Based on Standard Deviation of the Response and the Slope
Analytical Method Development
Noise LOD Signal to Noise = 3:1 LOQ Signal to Noise = 10:19/23/2013 76
• Berdasarkan kurva kalibrasi analit
Menurut Miller:
LOD = 3.S
Y/X+ yb
yb = intersep
LOQ = 10.S
Y/X+yb
( ˆ) / 2
2
/ y y N