www.elsevier.com / locate / bres
Research report
Neurotrophin-3 antisense oligonucleotide attenuates nerve
injury-induced A
b
-fibre sprouting
*
Deborah M. White
Department of Anaesthesia and Pain Management, University of Sydney, Royal North Shore Hospital, St. Leonards, N.S.W. 2065, Australia
Accepted 29 August 2000
Abstract
It is proposed that following peripheral nerve injury abnormal sprouting of Ab-fibre primary afferent neurons in the spinal cord contributes to the allodynia that often occurs with such injury. Allodynia is characterized as pain due to a stimulus which is normally non-noxious. Our recent in vivo experiments show that intrathecal administration of neurotrophin-3 (NT-3), in normal animals, induces allodynia and sprouting of Ab-fibres. In this study, we examine whether intrathecal administration of NT-3 antisense oligonucleotides (50
mM), via an osmotic pump for 14 days, attenuates nerve injury-induced sprouting and allodynia. The oligonucleotides used in this study were phosphorothioate modified and control experiments, using an ELISA, confirm that intrathecal administration of the antisense induces a significant decrease in NT-3 levels in the spinal cord. All surgery was conducted on anaesthetized Wistar rats (sodium pentobarbitone, i.p. 50 mg / kg). Consistent with previous studies, transganglionic labelling of Ab-fibres with choleragenoid-horseradish peroxidase (C-HRP) shows that complete transection of the sciatic nerve induces an expansion of C-HRP label into lamina II of the spinal dorsal horn. Using image analysis, we find that intrathecal administration of NT-3 antisense attenuates the density of C-HRP labelling in lamina II in nerve injured animals. A NT-3 sense oligonucleotide (50mM) has no effect. To test the effect of NT-3 antisense on allodynia, the nociceptive flexion reflex is examined, using an Ugo Basile Analgesymeter, in animals with partial sciatic nerve ligation. Intrathecal administration of 50mM NT-3 antisense significantly attenuates nerve injury-induced allodynia, whereas the sense oligonucleotide has no effect. These results provide further evidence that endogenous NT-3 contributes to both nerve injury-induced Ab-fibre sprouting and allodynia and demonstrates the potential of neurotrophin-3 antisense oligonucleotides as therapeutic agents for neuropathic pain. 2000 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Pain: pathways
Keywords: Neurotrophin-3; Antisense oligonucleotide; Ab-fiber; Allodynia; Nerve injury
1. Introduction central terminals of sensory neurons within the dorsal horn
of the spinal cord following nerve injury, which is Neuropathic pain, commonly associated with peripheral proposed to contribute to nerve injury-induced allodynia nerve injury, is characterized by spontaneous pain, in- [25,34]. In particular, Ab-fibres, which respond to non-creased responsiveness to painful stimuli (hyperalgesia) noxious stimuli, sprout from lamina III into lamina II, a and by the perception of normally non-noxious stimuli as region where small diameter unmyelinated C-fibre painful (allodynia). Multiple mechanisms presumably con- nociceptors normally terminate [33]. Ultrastructual studies tribute to the development and maintenance of neuropathic show that the sprouting terminals of Ab-fibre in lamina II pain and extensive research has been done to examine the form predominantly axodendritic synapses [3,35]. Electro-injury-induced plasticity of sensory neurons in an attempt physiological experiments indicate that these are functional to ascertain the mechanisms underlying such pain synapses as stimulation of injured Ab-fibres activates [6,29,34]. Of recent interest, is the reorganization of the lamina II neurons via either mono- or polysynaptic inputs
[13,36].
Recent studies from this laboratory have aimed to *Tel.:161-2-9926-8420; fax:161-2-9906-4079.
E-mail address: [email protected] (D.M. White). characterize the trophic factors contributing to the
ing of injured Ab-fibres [30–32]. Previously, we found animals showed normal behaviour upon recovery from the NPY acts indirectly via a release of neurotrophin-3 (NT-3) anaesthetic. Ethics approval for this study was obtained from spinal cord to enhance neurite outgrowth of disso- from RNSH / UTS Animal Ethics Committee in accordance ciated dorsal root ganglion (DRG) cells [30,32]. In vivo with the NH&MRC guidelines.
experiments further demonstrate that intrathecal
adminis-tration of NT-3 in normal animals induces allodynia and an 2.2. Oligonucleotides expansion of Ab-fibre terminals into lamina II [32]. The
role of endogenous NT-3, however, in nerve injury-in- The oligonucleotides used in this study were NT-3 duced Ab-fibre sprouting and allodynia has not been antisense, 59-CAT CAC CTT GTT CAC-39 and NT-3 determined. sense, 59-GTG AAC AAG GTG ATG-39 (Auspep, Aus-The aim of this transganglionic labelling study is to tralia) [26]. The oligonucleotides were fully phosphoro-examine the influence of intrathecal administration of NT- thioated and HPLC purified. To test the effectiveness of 3 antisense oligonucleotides on the sprouting of Ab-fibres NT-3 antisense to attenuate the synthesis of NT-3, 12.5ml following complete sciatic nerve transection. In addition, of 50mM of either antisense or sense oligonucleotide was behavioural studies were also conducted to test whether the administered intrathecally and 24 h later animals were NT-3 antisense oligonucleotide had an influence on al- overdosed with sodium pentobarbitone and samples of lodynia using animals with partial nerve ligation. spinal cord were removed. The tissue was weighed and stored at2808C until processed. The extraction procedure was according to a previous published method [37]. 2. Materials and methods Briefly, tissue samples were homogenized, by hand, in 3 ml of ice-cold 100 mM Tris–HCl buffer (pH 7.0) con-2.1. Surgery taining 2% bovine serum albumin, 1 M NaCl, 0.02% NaN , 4 mM EDTA, 0.2% Triton X-100, 53 mg / ml Catheters (18 cm of polyethylene tubing (PE-10)) were aprotinin, 0.5mg / ml antipain, 157 mg / ml benzamide, 0.1 implanted intrathecally by a method similar to that previ- mg / ml pepstatin A, 5.2 mg / ml phenylmethanesul-ously described [4]. Briefly, male Wistar rats (270–300 g; fonylfluoride. The homogenate was basified to pH 11 with Gore Hill Animal Research Laboratory, Sydney, Australia) 1 M NaOH and centrifuged at 13 0003g for 15 min at were anaesthetized (sodium pentobarbitone, 50 mg / kg; 48C. The supernatant was then acidified to pH 3 with 1 M intraperitoneal) and the tissue between the two posterior acetic acid and centrifuged for 30 min. The resulting articular processes of L5 was cleared and a laminectomy supernatant was neutralized with 1 M NaOH, centrifuged was performed to expose the spinal cord. The dura was and the final supernatant was assayed for NT-3 using a pierced using fine forceps and the catheter was gently fed commercially available ELISA kit (Promega). We found cephalad to a length of 2 cm. The catheter was flushed that the antisense induced a significant decrease in NT-3 with saline, containing 10 U / ml heparin, to ensure that levels in spinal cord 24 h after intrathecal treatment there was no leakage into the surrounding tissue. The (untreated, 24186286 pg / g wet tissue wt, n510; sense catheter was secured to the superficial lumbar muscles with treated, 21296156, n54; antisense treated, 13676340, 4 / 0 silk sutures and the remainder of the catheter was n56; untreated vs. antisense, P,0.05 and sense vs. tunnelled subcutaneously to a second skin incision midline antisense, P,0.05; Student’s t-test).
over the upper cervical region. Osmotic pumps (0.5ml / h;
14 days; Alzet, CA) were attached to the catheter and 2.3. Transganglionic labelling implanted subcutaneously either immediately, as for the
of 30% sucrose in PBS. The lumbar spinal cord was partially ligated and osmotic pumps were attached to the removed and stored overnight in 30% sucrose. Fifty catheters and implanted subcutaneously. The results are micron, transverse sections were cut on a freezing mi- expressed as the percentage change in mechanical thres-crotome. Sections were reacted for HRP using tetra- hold of the injured paw as compared to the sham-operated methylbenzidine as the chromagen, dehydrated, cleared control and calculated as: h(threshold of injured paw)2
and mounted. The density of terminal labelling across the (threshold of sham-operated paw)3100j/(threshold of different laminae was determined by image analysis. Five sham-operated paw).
groups of animals were examined. These were: (1)
un-treated controls; (2) sham operated; (3) nerve transection; 2.5. Statistics (4) nerve-transected animals treated intrathecally with
NT-3 antisense oligonucleotide; and (5) nerve-transected ani- Statistical analysis of the data was done using either mals treated intrathecally with NT-3 sense. The density of one-way analysis of variance (ANOVA) followed by labelling was determined by image analysis using a Scheffe test post hoc comparisons, or Student’s t-test.´ previously published method [1]. The image was captured
directly from the microscope using a JVC digital camera. Four boxes (25325 mm) were placed over the image
3. Results within lamina II and in the adjacent lamina III area. The
area of C-HRP label within these boxes was computed
3.1. Effect of NT-3 antisense oligonucleotide on nerve using Scion Image (Windows version of NIH Image; Scion
injury-induced Ab-fibre sprouting Corp., MA). An average of the four measurements was
calculated and the results are expressed as the ratio of
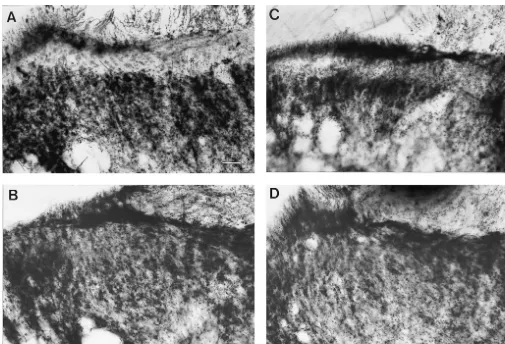
In sham-operated controls (n55), C-HRP label is pres-lamina II / pres-lamina III. Transganglionic labelling of Ab
-ent in laminae I, III and deeper lamina, but not pres-ent in fibres was also done on two groups of animals with partial
lamina II (Fig. 1A). This distribution of C-HRP is con-nerve ligation. In one group the C-HRP was injected
sistent with previous transganglionic labelling studies proximal to the ligature to label all Ab-fibres and, in the
using normal animals [1,35]. The image analysis data also second group, the conjugated was injected distal to the
shows that the density of C-HRP label in lamina II of ligature to label the intact fibres.
sham-operated animals is not different compared to un-In control experiments, the cross-sectional area of cells
treated animals (Fig. 2; n54). Two weeks following labelled with C-HRP was examined in L5 dorsal root
complete transection of the sciatic nerve (n55), C-HRP ganglion cells ipsilateral and contralateral to complete
label expands into lamina II (Fig. 1B) and the density is transection of the sciatic nerve (n54). Twenty-micron
significantly different from both untreated and sham rats serial sections were cut, mounted and processed as
de-(Fig. 2; ANOVA, P,0.05). scribed above. Cross-sectional area of individually labelled
Intrathecal administration of NT-3 antisense oligonu-cells was determined in every 20th section. The
circumfer-cleotide for 14 days via an osmotic minipump significantly ence of labelled cells, in which the nucleus could be seen,
attenuated the density of C-HRP label in lamina II of was manually drawn using a computer mouse and from
injured rats (n54) compared to the control group of this the cross-sectional area was computed.
animals with complete nerve transection (Figs. 1C and 2; nerve injury vs. nerve injury1antisense, P,0.05). Control 2.4. Behavioural test
experiments show that intrathecal administration of sense oligonucleotide (n54) had no effect on the nerve injury-The nociceptive flexion reflex was quantified using an
induced expansion of C-HRP into lamina II (Figs. 1D and Ugo Basile Analgesymeter (Comerio-Varese, Italy). A
2). mechanical force increasing linearly with time was applied
A comparison of the cross-sectional area of cells la-to the dorsum of the rat’s hindpaw by a dome-shaped
belled with C-HRP reveals there is no significant differ-plunger (diameter 1.4 mm, radius of curvature 368). The
ence in size of injured DRG cells compared to control cells nociceptive threshold was defined as the force, in grams, at 2
of the contralateral DRG (injured, 1062619 mm , 528 which the rat withdrew its paw. The chronic intrathecal 2
profiles, n54; control, 1086624mm , 404 profiles, n54). catheters were inserted 1 week before commencing
be-havioural studies to allow rats sufficient time for recovery.
Nociceptive thresholds were then determined on a daily 3.2. Effect of NT-3 antisense oligonucleotide on nerve basis over a 3-week period. Each day, thresholds were injury-induced allodynia
determined at 10-min intervals for a period of 2 h. The
Fig. 1. Photomicrographs of dorsal horn of lumbar spinal cord showing area of termination of Ab-fibres transganglionically labelled with C-HRP injected into the left sciatic nerve. (A) In sham-operated animals (n55), C-HRP label is present in lamina I, III and deeper laminae. No C-HRP label is present in lamina II (*). (B) Two weeks following complete sciatic nerve transection (n55), C-HRP label expands into lamina II. (C) Intrathecal administration of NT-3 antisense oligonucleotides via osmotic pumps for 14 days (n54) attenuated the intensity of C-HRP label in lamina II in nerve injured animals. (D) Intrathecal administration of NT-3 sense (n54) had no influence on intensity of C-HRP label in lamina II of rats with nerve injury. Calibration bar550
mm.
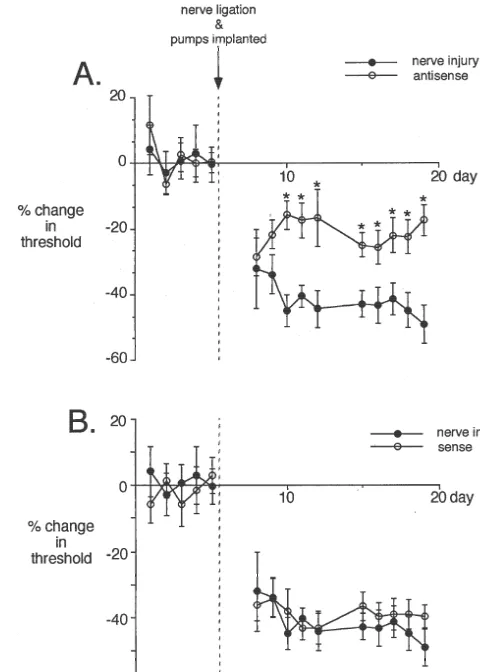
induces a significant decrease in threshold to mechanical C-HRP distal to the ligature also results in expansion of stimulation compared to measurements determined prior to label in lamina II, suggesting sprouting occurs in intact ligation in week 1 (Fig. 3; P,0.0002, n58). Animals Ab-fibres (Fig. 4C; n53).
treated intrathecally with NT-3 antisense significantly attenuated the nerve injury-induced allodynia compared to the untreated control group of animals (Fig. 3A; P,0.05,
n510). The analgesic effect of the NT-3 antisense became 4. Discussion significant 5 days after nerve injury and persisted for the
Fig. 2. Image analysis of the density of C-HRP in lamina II of the spinal cord. Two weeks after complete nerve transection (n55) there is a significant increase in the density of C-HRP in lamina II compared to both untreated (n54) and sham-operated animals (n55; P,0.05, ANOVA). Intrathecal administration of NT-3 sense oligonucleotide (50
mM; n54) had no effect on the C-HRP density in lamina II. The density of C-HRP in lamina II was significantly attenuated in nerve injured animals treated intrathecally with NT-3 antisense oligonucleotide (50
mM; n54; P,0.05). The results are expressed as a ratio of the area occupied by C-HRP-labelled nerve terminals in lamina II compared to lamina III.
repair and survival of injured neurons [5,7,17,20,22,27]. With respect to sensory neurons, exogenous NT-3 en-hances neurite outgrowth of dissociated DRG cells [2,18,32]; enhances regrowth of injured dorsal roots into the spinal cord in adult animals [20]; and accelerates the
regeneration of injured sciatic nerve [22]. Consistently, Fig. 3. Effect of intrathecally administered NT-3 antisense on nerve injury-induced allodynia. Partial nerve ligation induces a significant exogenous NT-3 also up-regulates the expression of
cyto-decrease in threshold to mechanical stimuli compared to the thresholds skeleton proteins such as MAP5 [21] and tubulin [18] in
measured prior to nerve injury (n58; P,0.0002, ANOVA). (A) Intrathec-dissociated sensory neurons. It has been suggested that
al administration of NT-3 antisense significantly attenuates the nerve NT-3 may be clinically useful for neuronal regeneration injury-induced allodynia (n510; P,0.05). The effect of NT-3 antisense after injury. Such an approach would be ill-advised in view becomes significant 5 days after nerve injury and osmotic pump implanta-tion. (B) Nerve injury-induced allodynia was not influenced by NT-3 of the present study showing that NT-3 contributes to
sense oligonucleotide (n510). aberrant neuronal growth and neuropathic pain.
Intrathecal administration of NT-3 antisense oligonu-cleotides only partially attenuates nerve injury-induced
current study, the transganglionic labelling experiments also show that rearrangement of the central terminals of Ab-fibres occurs in both intact and ligated sensory neu-rons. In view of the similarities between partial ligation and complete nerve transection it is tempting to speculate that the attenuation of the nerve injury-induced allodynia by NT-3 antisense is consistent with the suggestion that sprouting of Ab-fibres contributes to the allodynia. As there is no direct evidence showing sprouting of Ab-fibres induces allodynia other factors need to be considered. For example, NT-3 has been shown to enhance synaptic transmission of hippocampus neurons, which possibly involves a pre-synaptic mechanism and local protein synthesis [9,10]. NT-3 also weakly excites central neurons [11] and enhances spontaneous activity of cortical neurons putatively by attenuating GABA inhibitory input [12]. Although the actions of NT-3 on membrane potentials has not been examined in the spinal cord, increased sponta-neous activity, enhanced synaptic transmission and at-tenuation of inhibitory inputs are all plausible mechanisms that one would expect to contribute to neuropathic pain.
It was also observed that the NT-3 antisense signifi-cantly attenuated the allodynia 5 days after injury, which is before sprouting of Ab-fibres into lamina II is fully established [34,35]. A study by Shortland and Molander [24] provides evidence that Ab-fibres influence activity of lamina II neurons before sprouting occurs. Following nerve injury, stimulation of Ab-fibres induces the expres-sion of c-fos in lamina II as early as 3 days after injury [24]. How Ab-fibres influence the biology of neurons in lamina II at such an early time point is unknown. A possible explanation, is that mediators released from injured Ab-fibres diffuse into lamina II. This is exem-plified by the extensive diffusion throughout the dorsal horn of NPY following stimulation of Ab-fibres of an injured sciatic nerve [15]. Whether NT-3 contributes to allodynia via early changes in Ab-fibres that influence lamina II neurons needs to be examined.
Many studies have shown that exogenous NT-3 en-hances regrowth and survival of injured neurons in both the peripheral and central nervous systems. This study provides evidence that endogenous NT-3 contributes to the aberrant sprouting of Ab-fibres primary afferents and allodynia that occurs following peripheral nerve injury. The data further suggests that intrathecal administration of agents that attenuate the biological actions of NT-3 may potentially have therapeutic benefits in neuropathic pain. Fig. 4. Photomicrographs of C-HRP labelled nerve terminals in spinal
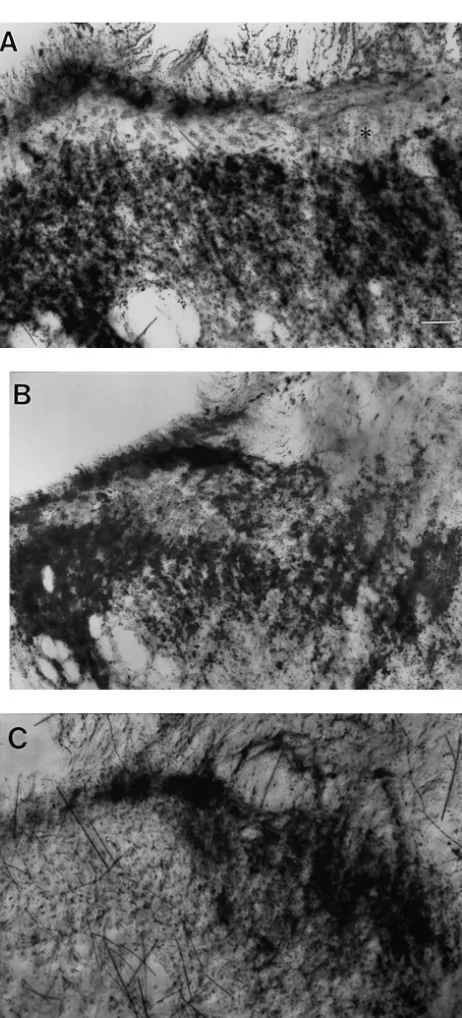
cord of rats with partial nerve ligation. (A) C-HRP injected into the left sciatic nerve of a sham-operated animals (same as Fig. 1A). C-HRP is
Acknowledgements present in lamina I, III and deeper lamina. There is no label in lamina II
(*). (B) C-HRP was injected into the left sciatic nerve proximal to the
ligature. There is an uneven density of C-HRP label across lamina II This project was funded by the Australian and New (n54). Calibration is same as in A. (C) Following injection of C-HRP
Zealand College of Anaesthetists and Northern Sydney distal to the ligature, there is a small area of label in lamina II, suggesting
Area Health Service. Thanks go to Dr Suellen Walker for intact neurons may also sprout into lamina II (n54). Calibration bar550
her support.
myelin-associated neurite outgrowth inhibitors, Exp. Neurol. 154 References
(1998) 583–594.
[18] L. Mohiuddin, K. Fernandez, D.R. Tomlinson, P. Fernyhough, Nerve [1] D.L.H. Bennett, J. French, J.V. Priestly, S.B. McMahon, NGF but not growth factor and neurotrophin 3 enhance neurite outgrowth and NT-3 or BDNF prevents the A fiber sprouting into lamina II of the up-regulate the levels of messenger RNA for growth-associated spinal cord that occurs following axotomy, Mol. Cell. Neurosci. 8 protein GAP-43 and Ta1 a-tubulin in cultured adult rat sensory
(1996) 211–220. neurons, Neurosci. Lett. 185 (1995) 20–23.
[2] P.A. Dijkhiuzen, W.T. Hermans, M.A. Teunis, J. Verhaagen, [19] C. Molander, G. Grant, Spinal cord projections from hindlimb Adenoviral vector-directed expression of neurotrophin-3 in rat muscle nerves in the rat studied by transganglionic transport of dorsal root ganglion explants results in a robust neurite outgrowth horseradish peroxidase, wheatgerm agglutinin conjugated horserad-response, J. Neurobiol. 33 (1997) 172–184. ish or horseradish peroxidase with dimethylsulfoxide, J. Comp. [3] T.P. Doubell, C.J. Woolf, Growth-associated protein 43 immuno- Neurol. 260 (1987) 246–255.
reactivity in the superficial dorsal horn of the rat spinal cord is [20] M. Oudega, T. Hagg, Neurotrophins promote regeneration of localized in atrophic C-fiber, and not in sprouted A-fiber, central sensory axons in the adult rat spinal cord, Brain Res. 818 (1999) terminals after peripheral nerve injury, J. Comp. Neurol. 386 (1997) 431–438.
111–118. [21] I. San Jose, E. Vazquez, N. Garcia-Atares, S. Rodriguez, J.A. Vega, J. [4] P.A.C. Durant, T.L. Yaksh, Epidural injections of bupivacaine, Represa, Expression of the cytoskeletal protein MAP5 and its morphine, fentanyl, lofentanil and DADL in chronically implanted regulation by neurotrophin 3 (NT-3) in the inner ear sensory rats: a pharmacological and pathological study, Anesthesiology 64 neurons, Anat. Embryol. 195 (1997) 299–310.
(1986) 43–53. [22] M. Shibayama, S. Hattori, B.T. Himes, M. Murray, A. Tessler, [5] M.J. Groves, S.F. An, B. Giometto, F. Scaravilli, Inhibition of Neurotrophin-3 prevents death of axotomized Clarke’s nucleus
sensory neuron apoptosis and prevention of loss by NT-3 adminis- neurons in adult rat, J. Comp. Neurol. 390 (1998) 102–111. tration following axotomy, Exp. Neurol. 155 (1999) 284–294. [23] Y. Shir, Z. Seltzer, A-fibers mediate touch-evoked allodynia and
¨
[6] T. Hokfelt, X. Zhang, Z. Wiesenfeld-Hallin, Messenger plasticity in hyperesthesia and C-fibers mediate thermal hyperalgesia in a rat primary sensory neurons following axotomy and its functional model of sympathetically-maintained neuropathic pain, Neurosci. implications, Trends Neurosci. 17 (1994) 22–29. Lett. 115 (1990) 62–67.
[7] D.A. Houweling, A.J. Lankhorst, W.H. Gispen, P.R. Bar, E.A.J. [24] P. Shortland, C. Molander, The time-course of Ab-evoked c-fos Joosten, Collagen containing neurotrophin-3 (NT-3) attracts regrow- expression in neurons of the dorsal horn and gracile nucleus after ing injured corticospinal axons in the adult rat spinal cord and peripheral nerve injury, Brain Res. 810 (1998) 288–293.
promotes partial functional recovery, Exp. Neurol. 153 (1998) 49– [25] P. Shortland, C.J. Woolf, Chronic peripheral nerve section results in
59. a rearrangement of the central axonal arborizations of axotomized
[8] R. Jenkins, S.B. McMahon, A.B. Bond, S.P. Hunt, Expression of A-beta primary afferent neurons in the rat spinal cord, J. Comp. cJun as a response to dorsal root and peripheral nerve section in Neurol. 330 (1993) 65–82.
damaged and adjacent intact primary sensory neurons in the rat, Eur. [26] H. Staecker, T.R. VanDe Water, P.P. Lefebvre, W. Liu, M. Moghadas-J. Neurosci. 5 (1993) 751–759. si, V. Galinovic-Schwartz, B. Malgrange, G. Moonen, NGF, BDNF [9] H. Kang, E.M. Schuman, Long-lasting neurotrophin-induced en- and NT-3 play unique roles in the in vitro development and hancement of synaptic transmission in the adult hippocampus, patterning of innervation of the mammalian inner ear, Dev. Brain Science 267 (1995) 1658–1662. Res. 92 (1996) 49–60.
[10] H. Kang, E.M. Schuman, A requirement for local protein synthesis [27] G.D. Sterne, R.A. Brown, C.J. Green, G. Terenghi G, Neurotrophin-in neurotrophNeurotrophin-in-Neurotrophin-induced hippocampal synaptic plasticity, Science 3 delivered locally via fibronectin mats enhances peripheral nerve 273 (1996) 1402–1406. regeneration, Eur. J. Neurosci. 9 (1997) 1388–1396.
[11] K. Kafitz, C.R. Rose, H. Thoenen, A. Konnerth, Neurotrophin- [28] Y.G. Tong, H.F. Wang, G. Ju, G. Grant, T. Hokfelt, X. Zhang, evoked rapid excitation through TrkB receptors, Nature 401 (1999) Increased uptake and transport of cholera toxin B-subunit in dorsal 918–921. root ganglion neurons after peripheral axotomy: possible implica-[12] H.G. Kim, T. Wang, P. Olafsson, B. Lu, Neurotrophin-3 potentiates tions for sensory sprouting, J. Comp. Neurol. 404 (1999) 143–158. neuronal activity and inhibits gamma-aminobutyratergic synaptic [29] S.G. Waxman, The molecular pathophysiology of pain: abnormal transmission in cortical neurons, Proc. Natl. Acad. Sci. USA 91 expression of sodium channel genes and its contributions to hyper-(1994) 12341–12345. excitability of primary sensory neurons, Pain 6 (suppl.) (1999) [13] I. Kohama, K. Ishikawa, J.D. Kocsis, Synaptic reorganization in the S133–140.
substantia gelatinosa after peripheral nerve neuroma formation: [30] D.M. White, K. Mansfield, Vasoactive intestinal polypeptide and aberrant innervation of lamina II neurons by Ab afferents, J. neuropeptide Y act indirectly to increase neurite outgrowth of Neurosci. 20 (2000) 1538–1549. dissociated dorsal root ganglion cells, Neuroscience 73 (1996) [14] W. Ma, M.A. Bisby, Partial and complete sciatic nerve injuries 881–887.
induce similar increases of neuropeptide Y and vasoactive intestinal [31] D.M. White, K. Mansfield, K. Kelleher, Increased neurite outgrowth peptide immunoreactivity in primary sensory neurons and their of cultured rat dorsal root ganglion cells following transection or central projections, Neuroscience 86 (1998) 1217–1234. inhibition of axonal transport of the sciatic nerve, Neurosci. Lett. [15] M.A. Mark, L.A. Colvin, A.W. Duggan, Spontaneous release of 208 (1996) 93–96.
immunoreactive neuropeptide Y from the central terminals of large [32] D.M. White, Contribution of neurotrophin-3 to the neuropeptide diameter primary afferents of rats with peripheral nerve injury, Y-induced increase in neurite outgrowth of rat dorsal root ganglion Neuroscience 83 (1998) 581–589. cells, Neuroscience 86 (1998) 257–263.
[16] S.B. McMahon, M.P. Armanini, L.H. Ling, H.S. Phillips, Expression [33] W.D. Willis, R.E. Coggeshall, Sensory Mechanisms of the Spinal and coexpression of Trk receptors in subpopulations of adult Cord, Plenum, New York, 1991.
primary sensory neurons projecting to identified peripheral targets, [34] C.J. Woolf, P. Shortland, R.E. Coggeshall, Peripheral nerve injury Neuron 12 (1994) 1161–1171. triggers central sprouting of myelinated afferents, Nature 355 (1992) [17] J. von Meyenburg, C. Brosamle, G.A.S. Metz, M.E. Schwab, 75–78.
primary afferents in the rat dorsal horn following peripheral [37] C. Zettler, D.C. Bridges, X.F. Zhou, R.A. Rush, Detection of axotomy, J. Comp. Neurol. 360 (1995) 121–134. increased tissue concentrations of nerve growth factor with an [36] M. Yoshimura, M. Okamoto, H. Baba, K. Shimoji, H. Higashi, improved extraction procedure, J. Neurosci. Res. 46 (1996) 581–
Plastic changes in synaptic transmission of rat dorsal horn neurons 594. following peripheral nerve transection, Soc. Neurosci. Abstr. 22