Brain Research 887 (2000) 191–193
www.elsevier.com / locate / bres
Short communication
Chronic stimulation of the peroneal nerve in rats upregulates the
pro-opiomelanocortin gene in spinal motoneurones
a b a ,
*
Sharon Hughes , Ruth A. Shiner , Margaret E. Smith
a
Division of Medical Sciences, Medical School, University of Birmingham, Birmingham B15 2TT, UK
b
School of Health Sciences, University of Wolverhampton, Wolverhampton, WV1 1SB, UK
Accepted 3 October 2000
Abstract
Continuous unilateral stimulation of the peroneal nerve in rats for 8 h per day for 2 or 7 days caused significant increases in POMC mRNA andb-endorphin immunoreactivity in both ipsilateral and contralateral motoneurones. Intermittent stimulation, for 10-min periods with 90-min rest periods, for 8 h per day for 2 days also caused upregulation of POMC mRNA. It is suggested that expression of POMC-derived peptides in motoneurones may be important for maintaining muscle contractile function. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters, and receptors
Topic: Peptides: anatomy and physiology
Keywords: b-Endorphin; Motoneuron; Pro-opiomelanocortin; Nerve stimulation; Exercise
b-Endorphin increases muscle contraction amplitude and ment [3]. It seems possible therefore that this peptide could decreases fatigue in isolated nerve–muscle preparations be induced during prolonged strenuous exercise when stimulated via the nerves [9], and stimulates glucose neuromuscular fatigue is present as a consequence of uptake in contracting muscles [2]. Furthermore b-endor- declining release of acetylcholine. In order to investigate phin receptors are present in normal adult muscles of this possibility the effect of chronic stimulation of the rodents [7]. This peptide, which is derived from pro- peroneal motor nerve, on the expression of both b -en-opiomelanocortin (POMC) is released from the pituitary dorphin immunoreactivity and POMC mRNA in the during exercise, and it may therefore have a role in the lumbar spinal cord was studied in adult rats. Part of this control of muscle function during exercise. However it can work has been published in abstract form [8].
be released from developing intramuscular motor nerves in Male Sprague–Dawley rats (|350 g body weight) were vitro by electrical stimulation of the nerves [4], and implanted with stainless steel, multi-stranded, coiled, tefl-therefore neuronally released b-endorphin may also be on-insulated, electrodes near the right lateral popliteal important in muscle function. nerve under aseptic conditions and 1–2% halothane Although b-endorphin immunoreactivity is normally (Fluothane ICI) anaesthesia, as described previously [1]. barely detectable in adult motoneurones [3,5,6], it is Electrical stimulation (0.3 ms pulse width, 10 Hz and up to expressed in conditions where neuromuscular function may 6 V) of the right peroneal nerve was started the day after be suboptimum such as congenital muscular dystrophy [3] the operation. The normal discharge frequency for and diabetes mellitus [7] which are characterised by the motoneurones innervating slow muscles is 10–20 Hz. The presence of secondary motor neuropathy, and in develop- electrodes were connected to Neurotech (Shannon, Ireland) stimulators via light-weight leads. Stimulation was con-tinuous (8 h each day), or intermittent (seven times a day, for periods of 10 min, with 90-min rest periods), for 2 or 7 *Corresponding author. Tel.:144-121-414-6903; fax: 1
44-121-414-days. In sham-operated animals the implanted electrodes 6919.
E-mail address: [email protected] (M.E. Smith). were not stimulated. Animals were killed by an overdose
192 S. Hughes et al. / Brain Research 887 (2000) 191 –193
of sodium pentobarbitone (Sagatal, RMB)|16 h after the first stimulation period.
The spinal cord was removed, washed thoroughly in phosphate-buffered saline (0.1 M) containing phenyl-methylsulphonyl fluoride (0.1 M) and cyclohexamide (0.1
mM), pH 7.4, and quickly frozen in isopentane cooled in liquid nitrogen. Serial cryostat sections (20mm thick, 10 to 12 per animal) were prepared from the lumbar segments, and examined for POMC mRNA and b-endorphin im-munoreactivity.
POMC transcript was detected by in situ hybridisation using a 24 base cDNA oligonucleotide antisense probe complementary to the ACTH 4–11 encoding region of rat POMC (Affiniti Research Products Ltd.). The corre-sponding sense probe was used in control experiments. The oligoprobes were covalently conjugated to calf intesti-nal alkaline phosphatase and the presence of POMC transcript was detected using the histochemical colorimet-ric method of McGadey [11] as described elsewhere [6].
Immunoreactivity was detected in every third section using an antibody to b-endorphin (Immuno-diagnostic Systems Ltd.) and the indirect peroxidase–antiperoxidase method as described previously [5]. Adjacent sections were stained with toluidine blue [10] to enable the total numbers of neurones to be counted. Only cells with a visible nucleus were included.
The results were expressed as the proportion of ventral horn motoneurones that expressed the POMC transcript or the peptide immunoreactivity. Statistical significance was
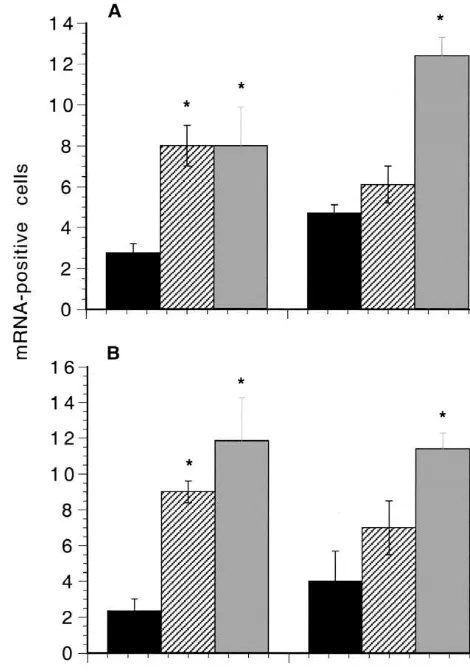
determined using ANOVA Fig. 1. (A) Effect of stimulation of the peroneal nerve on POMC mRNA In unoperated rats faint staining for POMC mRNA was expression in the ipsilateral spinal cord. (B) Effect of stimulation of the seen in a few cells, the proportion of cells being 4.060.6% peroneal nerve on POMC mRNA expression in the contralateral spinal cord. Black columns, sham operated (n55); hatched columns, intermittent (S.E.M., n53). The proportion of stained cells in
sham-stimulation (n53); grey columns, continuous stimulation (2 days, n54; 7 operated animals at 2 days or 7 days was not significantly
days, n56). The values are means6S.E.M. (bars). *Significant compared different. However in stimulated animals the staining was to sham-operated animals.
more intense, and was evident in a greater proportion of motoneurones. Fig. 1A compares the proportions on the
stimulated side in intermittently stimulated, continuously intermittently stimulated animals however, the proportion stimulated, and sham-operated rats (operated side) at 2 was significantly lower at 7 days than in the continuously days and 7 days. The proportions were significantly stimulated animals (P,0.02), and was not significantly (approximately threefold) higher at 2 days in both groups different from sham-operated animals.
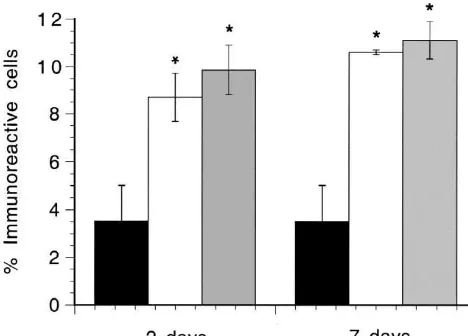
of stimulated animals compared to sham-operated animals Intense immunostaining for b-endorphin was seen in (P,0.02, in each case). At 7 days the proportion was over motoneurones in animals which had been stimulated fourfold higher in continuously stimulated animals than in continuously for 8 h per day for 2 or 7 days. Fig. 2 shows the sham-operated animals (P,0.01), and over twofold that the proportion of immunostained motoneurones was higher than in the intermittently stimulated animals (P, significantly increased on both sides of the spinal cord at 2 0.01). The value for the stimulated side at 7 days was and 7 days. The increases were of similar magnitude at the significantly higher than that for the stimulated side at 2 two time points.
S. Hughes et al. / Brain Research 887 (2000) 191 –193 193
tins, also influence muscle function (for a review see Ref. [12]), and these may act in concert with b-endorphin to maintain muscle function during exercise.
Acknowledgements
We are grateful to Professor O. Hudlicka for performing the surgical and stimulation procedures, and to the Well-come Trust for financial support.
References
[1] S. Egginton, O. Hudlicka, M. Glover, Fine structure of capillaries in ischaemic and non-ischaemic rat striated muscle. Effect of tor-Fig. 2. Effect of continuous stimulation on the expression ofb-endorphin
bafylline, Int. J. Microcirc. Clin. Exp. 12 (1993) 33–44. immunoreactivity in the lumbar spinal cord. Black columns, sham
[2] A.A.L. Evans, S. Khan, M.E. Smith, Evidence for a hormonal action operated (n55); open columns, stimulated side (2 days, n54; 7 days,
ofb-endorphin to increase glucose uptake in resting and contracting n56); dark grey columns, contralateral side. The values are
skeletal muscle, J. Endocrinol. 155 (1997) 387–392. means6S.E.M. (bars). *Significant compared to sham-operated animals.
[3] L.W. Haynes, M.E. Smith, Presence of immunoreactive a -melano-tropin and b-endorphin in spinal motoneurones of the dystrophic be an indirect effect due to activity in the sensory nerves mouse, Neurosci. Lett. 58 (1985) 13–18.
[4] L.W. Haynes, M.E. Smith, Release ofb-endorphin from the motor which are also stimulated by the procedure, or a feedback
nerve of the immature rat, J. Physiol. 351 (1984) 22P. effect from the contracting muscles.
[5] S. Hughes, M.E. Smith, Pro-opiomelanocortin-derived peptides in At 2 days the proportion of cells expressing POMC
transected and contralateral motor nerves of the rat, J. Chem. mRNA was similar in the intermittently stimulated and Neuroanat. 2 (1989) 227–237.
chronically stimulated rats. However in the intermittently [6] S. Hughes, M.E. Smith, Upregulation of the pro-opiomelanocortin gene in motoneurones after nerve section in mice, Mol. Brain Res. stimulated animals the proportion was lower at 7 days than
25 (1994) 41–49. at 2 days, although the difference between the two time
[7] S. Hughes, M.E. Smith, C.J. Bailey,b-Endorphin and corticotrophin points was not statistically significant. Moreover the
immunoreactivity and specific binding in the neuromuscular system proportion was significantly lower in intermittently stimu- of obese-diabetic mice, Neuroscience 48 (1992) 463–468. lated animals at 7 days than in chronically stimulated [8] S. Hughes, M.E. Smith, O. Hudlicka, Effect of peroneal nerve
stimulation in vivo in rats on the expression of the pro-animals at 7 days. Thus, some adaptation to the less severe
opiomelanocortin gene in spinal motoneurones, J. Physiol. 481P stimulation regime may have occurred by 7 days. The
(1994) 69P. increased expression in the contralateral motoneurones
[9] S. Khan, M.E. Smith, Effect of b-endorphin on the contractile may be due to a transneuronal mechanism [5] or the effect response in mouse skeletal muscle, Muscle Nerve 18 (1995) 1250– of a blood-borne influence from the stimulated muscles. 1256.
[10] K. Kramer, G.M. Windrum, The metachromatic staining reaction, J.
b-Endorphin may be released at the neuromuscular
Histochem. Cytochem. 3 (1955) 227–237. junction during muscle contraction [4] to augment the
[11] J. McGadey, A tetrazolium method for non-specific alkaline phos-effect of the circulating peptide during exercise, when it
phatase, Histochemie 23 (1970) 180–184.
could help to maintain the contractile strength of the [12] F.L. Strand, K.J. Rose, L.A. Zuccarelli, J. Kume, S.E. Alves, F.J. muscles [9] and promote glucose utilisation [2]. Interest- Antonowich, L.Y. Garrett, Neuropeptide hormones as neurotrophic