www.elsevier.com/locate/ibmb
Do thyroid hormones function in insects?
K.G. Davey
*Department of Biology, York University, Toronto, Ont. M3J 1P3, Canada
Received 31 October 1999; received in revised form 31 December 1999; accepted 23 January 2000
Abstract
Earlier work demonstrated that phenoxy–phenyl compounds such as fenoxycarb and thyroxine mimicked the effects of JH III in causing a reduction in volume of the follicle cells of Locusta migratoria. While these compounds were only moderately effective, a derivative of thyroxine, 3,39,5-triiodothyronine (T3) was as effective as JH III, and T3 has been shown to bind to the same membrane receptor and activate the same pathway as JH III. The current paper shows that other thyroxine derivatives vary in activity. 3,39,59-Triiodothyronine (reverse T3) is inactive. 3,5-Diiodothyronine (T2) is more active than JH III, while its relatives (iodines at 39,59or at 3,39) are inactive. When follicles are exposed in vitro to rhodamine conjugated T3, the fluorescent compound can be seen to enter the cells and accumulate there: this process is inhibited by cycloheximide or by a temperature of 0°C. The accumulation is antagonised by JH III but not JH I (which does not bind to the JH III membrane receptor) and by an antiserum raised against the putative membrane receptor protein. The action of T3, but not T2, is inhibited by 6-n-propyl-2-thiouracil or by aurothioglucose, both known to inhibit deiodinases. The activity of T3, but not of T2, increases with time of exposure to the follicle cells. These facts suggest that T3 enters the cells by receptor mediated endocytosis and is converted to a more active compound. Immunoreactivity to T3, but not thyroxine, can be detected in the haemolymph of locusts, and the titre varies slightly with the gonotrophic cycle. The food shows immunoreactivity for both thyroxine and T3. These findings suggest that thyroid hormones are ingested by locusts and have the potential to be used as hormonal signals in the control of egg production.2000 Elsevier Science Ltd. All rights reserved.
Keywords: Juvenile hormone; Receptors; Thyroid hormones; Endocytosis; Deiodinase
1. Introduction
While the thyroid hormones are customarily regarded as vertebrate hormones, evidence is accumulating that derivatives of thyroxine may have a role to play in the development of a variety of invertebrates. Eales (1997), in a provocative review, has argued that derivatives of thyroxine, originating in the food of many invertebrates, may function in the control of development as vitamins. In insects, feeding 3,5,39,59-tetraiodothyronine (T4) to silkworms, has a number of effects on silk production and reproductive function, which were interpreted as indirect, acting perhaps via ecdysone (Thagaraja et al. 1991, 1993). Others have documented a variety of long lasting effects of a single injection of T4 into larvae of Bombyx mori or Antherea mylitta, including protein and
* Tel.:+1-416-736-2100 x33804; fax:+1-416-736-5698.
E-mail address: [email protected] (K.G. Davey).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 6 1 - 8
nucleic acid and glycogen content of fat body (Chaudhuri et al. 1986, 1992; Chaudhuri et al., 1987a,b), protein and nucleic acid turnover (Reddy et al., 1994a,b), haemolymph amino acid content (Reddy et al., 1994c) and ion-specific ATPases (Reddy et al. 1994d, 1996). T4 also stimulates the release of triglycerides and fatty acids from the fat body of Hyalophora cecropia in vitro (Bhakthan and Gilbert, 1968).
In most insects, the vitellogenin gains access to the oocyte surface via spaces between the cells of the fol-licular epithelium, and in many species, the appearance of these open spaces is governed by JH. The follicle cells respond to JH by shrinking and by altering the cytoskele-ton and the distribution of septate junctions, resulting in the appearance of large lateral spaces between the cells (Davey, 1996). These effects are mediated by a mem-brane receptor (Sevala et al., 1995). The effect of JH on cell volume involves the activation of Na+/K+ ATPase (Davey, 1996).
com-pounds such as fenoxycarb mimic these effects. We have shown that both T4 and particularly 39 ,3,5-triiodothyron-ine (T3), when applied to follicle cells of locusts in vitro, induce the same rapid reduction in cell volume as JH III, and this effect is inhibited by ouabain, implicating Na+/K+ ATPase (Davey and Gordon, 1996). More recently, we have shown that T3 also mimics the action of JH I in causing both a reduction in volume and an increase in patency in the follicle cells of Rhodnius pro-lixus. Moreover, the effects of T3 on the follicle cells of L. migratoria appear to be mediated by the same recep-tor as JH III. The effect on follicle cell volume is blocked by incubation in an antibody raised against the 35 kDa binding protein for JH III, T3 shows specific and satu-rable binding to membrane preparations of follicle cells, and JH III will compete for these binding sites (Kim et al., 1999).
In this paper, I further explore the effects of deriva-tives of thyroxine on follicle cells, and examine the occurrence and probable origin in locusts of immunore-active thyroxine derivatives.
2. Materials and methods
2.1. Insects
Locusts were obtained from a large colony maintained on a 12:12 light:dark cycle with the temperature at 36°C during the day and at 22°C during the night.
2.2. Chemicals
39,59,3-Triiodothyronine, 3,39-diiodothyronine and 39,59-diiodothyronine were purchased from Henning (Berlin). All other chemicals were from Sigma (St. Louis, MO, USA).
Rhodamine conjugated T3 (rho-T3) was prepared and purified according to Cheng et al. (1980). This material has been shown in mammals to bind only to those sites to which T3 binds (Cheng et al., 1980).
2.3. Changes in cell volume
Suspensions of follicle cells of ovaries in mid to late vitellogenesis were prepared as previously described (Davey and Gordon, 1996). Changes in cell volume were detected by measuring the optical path difference (OPD) by quantitative interference microscopy as described in Davey and Gordon (1996). Changes in the OPD under the conditions used in this study are inversely pro-portional to changes in cell volume, so that an increase in OPD indicates a decrease in cell volume. In practice, measurements for any particular hormone were perfor-med on 25 cells selected at random. The results presented in each graph were performed on a suspension
prepared from a single ovary. Each graph thus represents results from a single experiment: all experiments have been replicated at least three times with qualitatively identical results in terms of the differences in OPD among treatments, although the control values varied among individual locusts. Unless otherwise indicated, the cells were exposed to the various test solutions for 30 min before the OPD was measured.
2.4. Uptake of rho-T3
Uptake of rho-T3 by the follicular epithelium was measured by fluorescence microscopy. Individual ovari-oles were dissected from locust ovaries in a medium con-sisting of 40% Eagle’s Medium in Schneider’s Droso-phila Medium (Gibco, Grand Island, NY, USA), and their connective tissue sheaths removed. For any one experiment, ovarioles from a single female were used. In a typical experiment, six to seven ovarioles were immersed in 3 ml of medium containing 1.0µM rho-T3 at room temperature for 60 min. They were then rinsed quickly in medium, and mounted in medium on a micro-scope slide under a coverslip for viewing and measure-ment by epifluorescence. In some experimeasure-ments, follicles were pre-incubated in inhibitors or an antibody raised against the JH III membrane receptor (Kim et al., 1999) for 30 min before exposure to rho-T3.
Fluorescence was measured using a Zeiss Photomicro-scope III with an attached photometer model 01 K with photomultiplier R446. Illumination was from a mercury lamp HBO 100 W/2 driven by a stabilised DC power supply, and the light path was chopped by a slotted disk at 60 Hz. The epi-illumination dichroic mirror/filter set was #31002 (D 540/25 mirror, 565 DCLP filter) from Chroma Technology Corporation (Brattleboro, Vermont, USA). The photometer was adjusted so as to read 0 when no specimen was in the field, and a follicle which had not been exposed to the rho-T3 also yielded a reading of 0. For measurement, a field of follicle cells on the terminal follicle of an ovariole was centred and focused under tungsten illumination, and then switched to fluor-escence, and the measurement taken immediately in order to avoid the rapid photo bleaching of the specimen. This was repeated for five fields on each of at least five follicles, using a 40× Neofluar objective. The readings for each ovariole were typically within 10% of one another and the average was taken as the reading for a follicle. The data are reported as means of at least five follicles. The photometer is very sensitive, detecting the very weak fluorescence produced by this procedure.
2.5. Radioimmunoassay (RIA)
6-n-propyl-2-thiouracil (PTU) to inhibit deiodinases. The supernatant remaining after centrifuging at 1500g was used for RIA. For more quantitative determinations, tissues or food were homogenised in 3 ml of phosphate buffer containing PTU and 2.5 mg pronase. The homo-genate was incubated at 37°C for 12 h, and then 1.5 ml of NH4OH were added. The supernatant from centrifug-ation at 1500g was evaporated to dryness and the residue taken up in 1 ml of distilled water for processing by RIA. Haemolymph was not extracted prior to assay.
RIA was performed using commercial kits (Inter-med-ico, Markham, Ont.) for total T4 and T3. The standards supplied in the kit were used for most samples. Dilution curves of gut contents and food were linear and parallel to the standard curves. For quantitation of haemolymph T3, a standard was prepared based on locust haemo-lymph. Twenty millilitres of haemolymph were extracted from locusts through a small incision in the cervical membrane. A small quantity (a few crystals) of phenyl thiourea to inhibit darkening, and 0.1% sodium azide to inhibit bacterial growth were added. The haemolymph was subjected to extraction with activated charcoal fol-lowed by centrifugation at 1550g to remove any T3, and the resulting supernatant used as a solvent in preparing the standards of T3.
3. Results
3.1. The action of other thyroid hormone derivatives on cell volume
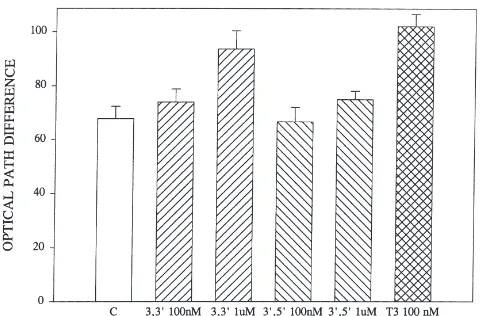
While T3 is active in inducing reduction in cell vol-ume, 39,59,3-triiodothyronine (reverse T3, rT3) is devoid of activity at all concentrations tested (Fig. 1). However, T2 is extraordinarily active, causing significant (P,0.02) reductions in cell volume at concentrations as low as
Fig. 1. The effect of reverse T3 on the optical path difference (here expressed as degrees of rotation of the polarising analyser) of follicle cells in vitro. Each bar represents the mean of determinations on 25 cells, and the vertical lines indicate the standard error of the mean.
Fig. 2. The effect of T2 (3,5-diiodothyronine) on the optical path difference of follicle cells in vitro. Data treated as in Fig. 1.
0.01 nM, with optimal activity appearing at 0.1–1.0 nM, two orders of magnitude lower than the optimum of 100 nM for JH III or T3 (Fig. 2). Only two other diiodo derivatives are available. One of these, 3,39 -diiodothy-ronine, is weakly active, producing a significant increase in optical path difference only at 1.0 µM (P,0.01), while the other derivative (39,59-diiodothyronine) is inactive (Fig. 3). A precursor of T4, 3,5-diiodotyrosine, is also inactive (data not shown).
3.2. The uptake of rho-T3
When follicle cells are exposed in vitro to rho-T3 at
1µM, the cells contain fluorescent material when
exam-ined after 1 h by epifluorescence microscopy. This flu-orescence is too weak to photograph, but the fluor-escence can be detected by confocal microscopy, where
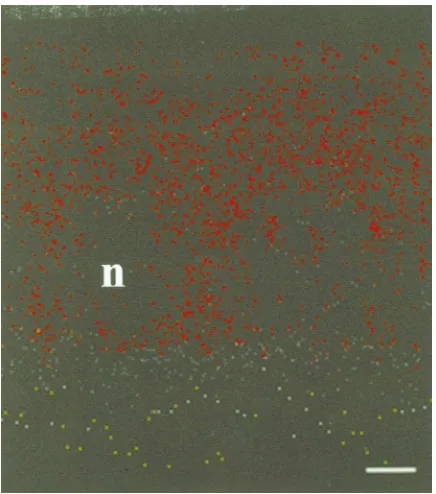
Fig. 4. Confocal image of a vertical section derived from a z series through the follicular epithelium of an ovariole exposed to 1µM rho-T3 for 90 min. The basal (haemocoel) side of the epithelium is at the top of the photograph. The clear spaces (indicated by n) represent the nuclei of the cells. The line represents 3µm.
the rho-T3 is seen to be in the form of vesicles (Fig. 4). While the fluorescence in a conventional microscope is weak, it can be detected by the fluorometer attached to the microscope. Fig. 5 depicts a typical result of an experiment in which follicles were exposed to rho-T3 at room temperature and on ice, and were assessed 1 h later. In the same experiment some follicles were
pre-Fig. 5. The fluorescence of follicular epithelium exposed in vitro to 1µM rho-T3 for 60 min. C, control; ICE, exposed at 0°C; CHX, pre-incubated in 1 mM cycloheximide for 30 min before exposure to rho-T3 at room temperature. The fluorescence units are arbitrary, with the settings on the photometer adjusted so as to give a zero reading for epithelium not exposed to rho-T3. Each bar represents the means of determinations made on at least five follicles, and the vertical lines indicate the standard error of the mean.
Fig. 6. The fluorescence of follicular epithelium exposed to 1 µM rho-T3 for 60 min (control) or pre-incubated in 100 nM JH III or JH I. See Fig. 5 for details.
incubated for 30 min in 1 mM cycloheximide and then placed in cycloheximide and rho-T3 for 60 min. While those follicles exposed at room temperature contained abundant fluorescence, those exposed at 0°C showed very little uptake. Incubation with cycloheximide also blocked uptake.
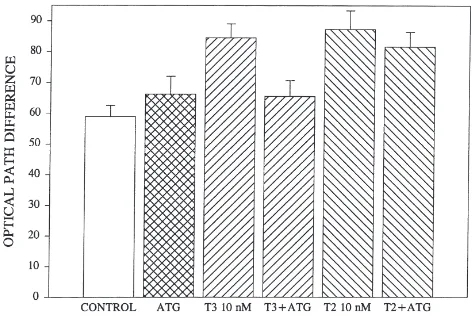
When follicles are exposed to rho-T3 in the presence of JH III at 100 nM, uptake is markedly inhibited (P,0.01) but when JH I is included as the competing ligand, there is no effect (Fig. 6). When the follicles are pre-incubated in an antibody raised in rabbits against the 35 kDa membrane binding protein for JH III for 30 min before adding rho-T3, uptake is markedly less (P,0.001) than for follicles not exposed to the antibody (Fig. 7). Although non-immune serum was not included in the protocol of this experiment, separate experiments using non-immune rabbit serum detected no effect on the uptake of rho-T3 or on the JH or T3 stimulation of the OPD (data not shown).
Fig. 8. The effect of pre-incubation in 1µM 6-n-propyl-2-thiouracil on the stimulation by T3 or T2 of the optical path difference of follicle cells in vitro. Data treated as in Fig. 1.
3.3. The effect of inhibitors of 59-deiodinase
When follicle cells are pre-incubated in 1 µM PTU for 30 min before being exposed to T3 or T2, the capacity for T3 to induce a reduction in cell volume is eliminated, while T2 retains its capacity, albeit at a reduced level, to cause a significant (P,0.01) increase in OPD (Fig. 8). Similarly, exposure of follicle cells to aurothioglucose at 100 nM inhibits the action of T3 (P,0.05), but not that of T2 (Fig. 9).
3.4. The effect of time on the action of T3 and T2
We have not previously timed carefully the interval between application of a ligand and the assessment of its effect on the optical path difference. When T3 is added to a suspension of follicle cells and the effect mea-sured as quickly as possible (within 10 min), its effect on the optical path difference is small, whereas incu-bation for 60 or 120 min before the effect is assessed
Fig. 9. The effect of pre-incubation in 100 nM aurothioglucose on the stimulation by T3 or T2 of the optical path difference of follicle cells in vitro. Data treated as in Fig. 1.
Fig. 10. The effect on the optical path difference of exposure of fol-licle cells in vitro to 10 nM T3 or T2 for varying lengths of time (indicated in min). Data treated as in Fig. 1.
produces a greater effect (P,0.01 for 10 min vs. 60 min). With T2, however, the effect after 10 min is not different from the effect after 60 min (P.0.1) (Fig. 10).
3.5. The effect of cycloheximide on the action of T3 and T2
Because cycloheximide blocks the uptake of rho-T3, we examined its effect on the action of T3 and JH III. The cells were pre-incubated for 30 min in 1 mM cycloheximide before adding the hormones. The effect on the OPD was assessed 30 min later. The effect of T3 is abolished by the inhibitor, whereas the effect of JH III was undiminished (Fig. 11).
3.6. The occurrence of immunoreactive T3 and T4
Extracts of ovaries, brains, thoracic muscles, fat bod-ies and digestive system were tested by RIA for T4 and T3. Of these only gut (particularly mid-gut) yielded
sistently positive results. In three separate quantitative determinations, there were 80–270 (mean 163) pg of T3 equivalents per gut and 8–10.5 (mean 9.0)µg T4 equiva-lents per gut.
When the gut tissue and the gut contents were assayed separately, only gut contents displayed any immunoreac-tivity. When food was extracted, wheat shoots were found to contain 28 ng of T3 and 1.8 µg T4 equivalent activity per gram fresh weight, while bran contained 23
µg of T3 and 243 µg of T4.
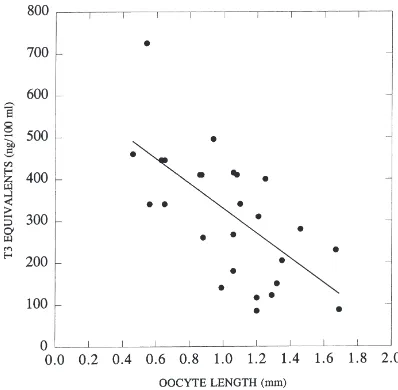
Only T3 immunoreactivity is detected in haemo-lymph. Its titre varies to some degree with the gono-trophic cycle of the female (Fig. 12).
4. Discussion
The data presented in this paper raise a number of questions. The first and most obvious is the possibility that the thyroid hormones may have a functional role in insects. The experimental results are all consistent with a scheme which sees T3 and T4 enter the insect with the food. From the haemolymph, T3 at least, enters the target cells by receptor mediated endocytosis, and is there converted by a deiodinase to T2, which then exerts a powerful effect on the cells. This possible mechanism is similar to that which occurs in vertebrates, where both T3 and T4 enter cells by receptor mediated endocytosis. In the liver T4 is deiodinated to T3, the active form of the hormone (Di Liegro et al., 1987). The parallel is even closer in terms of the effect of T3 on mitochondrial metabolism in liver. The action of T3 is blocked by inhibitors of deiodinase, whereas the effect of T2 is not (Horst et al., 1989).
Fig. 12. The titres of T3 equivalents detected in the haemolymph of individual female locusts in various stages of the gonotrophic cycle as indicated by the length of their terminal oocytes. Based on samples of at least 100µl removed from each insect before dissection to determine the oocyte length. The r2for the regression line is 0.43.
However, great caution ought to be used in the interpretation of the essentially pharmacological experi-ments presented here. While it is clear from the results of the uptake and time of exposure experiments that T3 enters the cell and is converted from an inactive to an active form, and while the direct effect of T2 and the results of the experiments involving PTU and aurothiog-lucose suggest strongly that the T3 is deiodinated to T2, there is no direct evidence that this is the case. It is important to note that the enzymatic conversion of T3 to T2 has never been shown to occur in any vertebrate (McLeese and Eales, 1996). While PTU is widely used as an inhibitor of deiodinases, it is also known to affect other events. Both aurothioglucose and PTU are thought to owe their action to their ability to interact with the selenocysteine of deiodinases. This view has been ques-tioned, and, in any case, other enzymes also contain selenocysteine groups (Stadtman, 1996). More definitive results would involve a direct demonstration of deiodin-ase activity and the chemical characterisation of the immunoreactive materials found in the haemolymph as well as other thyoxine derivatives in insects. These experiments are currently in progress.
by the cell. The question of the possible uptake of JH into the follicle cells is an important one, which is treated more extensively in another paper (Davey, 2000).
It is of some interest that there was considerable vari-ation in the capacity of various suspensions of follicle cells to take up rho-T3. For example, the control level in the suspensions used in Fig. 6 is very different from that in Fig. 7. While each suspension is made from the cells in a single female, the stage of development of the ovaries may differ from female to female. This may indi-cate that the capacity to take up rho-T3 varies through the gonotrophic cycle.
The significance of the uptake of T3 may lie else-where. It is possible, for example, that the uptake is sim-ply a means of detoxifying an exotic material. Prelimi-nary experiments have not suggested that other tissues such as fat body also take up the ligand. Alternatively, the uptake may represent an entirely normal process of receptor turnover.
T4 and some of its derivatives have been reported in tissues of Periplaneta americana, based on seperation by paper chromatography of products obtained after injection of radioiodine (Limpel and Caseda, 1957), but more extensive analysis by Tong and Chaikoff (1961) found only iodinated tyrosine derivatives, with no sign of any of the thyroid hormones. The fact that thyroxine derivatives are processed by insects is scarcely surpris-ing, given the importance of tyrosine in insect cuticle formation.
The levels of T3 immunoreactivity found in the hae-molymph of females is in the range of 1-10 nM. Maxi-mal stimulation of the follicle cells was earlier reported as requiring 100 nM (Davey and Gordon, 1996). How-ever, interpretation of these observations is rendered problematical by two observations reported in the cur-rent paper. First, T3 is taken up by the cells. Second, the activity of T3 depends on this uptake process. When the process is blocked, the T3 is inactive, or nearly so. Moreover, the effect of a dose of 10 nM T3 is greatly enhanced by incubation for 60 min. Thus, the titres detected by RIA have the potential to be physiologi-cally significant.
Whether or not this potential is realised remains to be seen. It would be a mistake to conclude at this stage that thyroid hormones are important to egg production or any other physiological process in the locust. It is worth remembering that removal of the corpus allaturn, the source of juvenile hormone, prevents egg production (Girardie and Girardie, 1996). If thyroid hormones have a function in locust reproduction, it will surely be as a supplement to JH. Perhaps it forms a signal indicating the quantity of food digested, thereby increasing the number of eggs produced.
Acknowledgements
Research in my laboratory is supported by the Natural Sciences and Engineering Research Council of Canada. I am grateful to Brian Gordon for technical assistance, to Professor B.G. Loughton for supplying the locusts, to Elisabeth Hanson, who did most of the RIAs, and to Professor J.G. Eales of the University of Manitoba for advice about thyroid hormones.
References
Bhakthan, N.M.G., Gilbert, L.I., 1968. Effects of some vertebrate hor-mones on lipid mobilization in the insect fat body. Gen. Comp. Endocrinol. 11, 186–197.
Chaudhuri, A., Medda, A.K., 1986. Changes in protein and nucleic acid contents of male gonad of silkworm, Bombyx mori at different developmental stages after after thyroxine treatment. Proc. Natl. Acad. Sci. India 56, 301–306.
Chaudhuri, A., Medda, A.K., 1987a. Thyroxine induced alterations in protein and nucleic acid content of fat body of female silkworm, during different developmental stages. Insect Sci. Appl. 8, 43–48. Chaudhuri, A., Medda, A.K., 1987b. Effect of thyroxine on protein, RNA and DNA content of ovary of silkworm, Bombyx mori, at larval, pupal and adult stages of development and production of eggs. Zool. Jahrb. Anat. 115, 85–90.
Chaudhuri, A., Medda, A.K., 1992. Thyroxine induced alterations in glycogen content of fat body of female silkworms Bombyx mori (race nistari) during larval, pupal and adult stages of development. Ann. Entomol. 10, 17–21.
Cheng, S.Y., Maxfield, F.R., Robbins, J., Willingham, M.C., Pastan, I.H., 1980. Receptor-mediated uptake of 3,39,5-triiodo-thyronine by cultured fibroblasts. Proc. Natl. Acad. Sci. USA 77, 3425–3429. Davey, K.G., 1996. Hormone control of the follicular epithelium
dur-ing vitellogenin uptake. Invert. Reprod. Develop. 30, 249–254. Davey, K.G., 2000. The modes of action of juvenile hormones: some
questions we ought to ask. Insect Biochem. Mol. Biol. 30, 663–669. Davey, K.G., Gordon, D.R.B., 1996. Fenoxycarb and thyroid hormones have JH-like effects on the follicle cells of Locusta migratoria in vitro. Arch. Insect Biochem. Physiol. 32, 613–622.
Di Liegro, I., Savettieri, G., Cestelli, A., 1987. Cellular mechanism of thyroid hormones. Differentiation 35, 165–175.
Eales, J.G., 1997. Iodine metabolism and thyroid-related functions in organisms lacking thyroid follicles: Are thyroid hormones also vit-amins? P.S.E.B.M. 214, 302–317.
Horst, C., Rokos, H., Seitz, H.J., 1989. Rapid stimulation of hepatic oxygen consumption by 3,5-di-iodo-l-thyronine. Biochem. J. 261, 945–950.
Kim, Y., Davari, E.D., Sevala, V., Davey, K.G., 1999. Functional bind-ing of a vertebrate hormone, l-3,5,39-triiodothyronine (T3), on insect follicle cell membranes. Insect Biochem. Mol. Biol. 29, 943–950.
Limpel, L.E., Caseda, J.E., 1957. Iodine metabolism in insects. I. In vivo metabolism of radionuclide. J. Exp. Zool. 135, 19–27. McLeese, J.M., Eales, J.G., 1996. 3,5,39-triiodo-l-thyronine andl
-thy-roxine uptake into red blood cells of rainbow trout, Oncorhynchus
mykiss. Gen. Comp. Endocrinol. 102, 47–55.
Reddy, K.D., Chaudhuri, A., Sukumar, K., 1994a. Thyroxine effect on protein and nucleic acid turnover of testis and ovary during 5th larval stage of non-diapausing tasar silkworm, Antheraea mylitta (Saturniidae: Lepidoptera). Indian J. Exp. Biol. 32, 409–412. Reddy, K.D., Chaudhuri, A., Sukumar, K., 1994b. Effect of
fat body during 5th larval stage of non-diapausing tasar silkworm,
Antheraea mylitta (Saturniidae: Lepidoptera). Indian J. Exp. Biol.
32, 413–417.
Reddy, K.D., Chaudhuri, A., Sukumar, K., 1994c. l-thyroxine (T4) elevates the the free amino acid pool of haemolymph plasnia of tasar silkworm, Antheraea mylitta (Saturniidae: Lepidoptera). Horm. Metab. Res. 26, 570–573.
Reddy, K.D., Chaudhuri, A., Sukumar, K., 1994d. Enrichment of ion specific adenosine triphosphate activities by thyroxine in different tissues of the silkworm, Bombyx mori L., during insect develop-ment. Insect Biochem. Mol. Biol. 24, 243–248.
Reddy, K.D., Chaudhuri, A., Thangavelu, K., 1996. Influence of thy-roxine on different ion-dependent ATPase activities in fat body of Tasar silkworm, Antherea mylitta D. Gen. Comp. Endocrinol. 194, 20–28.
Sevala, V.L., Davey, K.G., Prestwich, G.D., 1995. Photoaffinity
labe-ling and characterization of a juvenile hormone binding protein in the membranes of follicle cells of Locusta migratoria. Insect Biochem. Mol. Biol. 25, 267–273.
Stadtman, T.C., 1996. Selenocysteine. Annu. Rev. Biochem. 65, 83– 100.
Thagaraja, B.S., Kelly, T.J., Masler, E.P., Borkovec, A.B., 1991. Thy-roxine-induced haemolymph protein and ecdysteroid increases in the silkworm, Bombyx mori L.: Effect on larval growth and silk production. J. Insect Physiol. 37, 153–159.
Thagaraja, B.S., Kelly, T.J., Masler, E.P., Borkovec, A.B., 1993. Thy-roxine-induced changes in ovarian protein and ecdysteroid increases in the silkworm, Bombyx mori L.: Effect on ovarian matu-ration and egg production. Comp. Biochem. Physiol. 104A, 247– 253.
Tong, W., Chaikoff, I.L., 1961.131I utilization by the aquarium snail