www.elsevier.com/locate/ibmb
Injection of Dip-allatostatin or Dip-allatostatin pseudopeptides into
mated female Diploptera punctata inhibits endogenous rates of JH
biosynthesis and basal oocyte growth
Chris S. Garside
a, Ronald J. Nachman
b, Stephen S. Tobe
a,*aDepartment of Zoology, University of Toronto, Toronto, On, M5S 3G5, Canada
bVeterinary Entomology Research Unit, Southern Plains Area Research Center, ARS, US Department of Agriculture, 2881 F&B Road, College
Station, TX 77845, USA
Received 31 October 1999; received in revised form 31 December 1999; accepted 25 January 2000
Abstract
Studies on the catabolism of allatostatins (ASTs) provided the rationale for the design of a series of Dip-allatostatin-derived pseudopeptide mimetic analogues. In vitro, the Dip-ASTs and pseudopeptides show varying degrees of resistance to catabolism and all show significant inhibition of juvenile hormone (JH) biosynthesis. This study was undertaken to determine whether potent Dip-ASTs and/or their pseudopeptide mimetic counterparts caused ‘allatostatic’ effects in vivo following injection into mated female Diploptera punctata. Animals injected with aqueous solvent or Dip-AST 7(1–7) N-terminal fragment, which excludes the active core region of the ASTs, were used as controls. An in vitro radiochemical assay revealed that injection of Dip-AST 5, 7 or pseudopeptide analogues 397-2 or AST(b)φ2 significantly inhibited the biosynthesis of JH (P,0.05). The results also indicate that basal oocyte growth was significantly inhibited by injection of these same compounds, with the exception of Dip-AST 7 (P,0.05). Analogues 396-1 and 419 did not significantly inhibit rates of JH biosynthesis but did significantly inhibit the growth of basal oocytes. Analyses of feeding, excretion and food absorption/utilization patterns of these same animals suggested that these com-pounds are not toxic to the insect; rather they directly inhibit the biosynthesis of JH by the corpora allata, and reduce the rate of growth of basal oocytes. Disruption of critical reproductive and/or developmental processes by pseudopeptide analogues of the ASTs could provide novel and selective strategies for future insect pest management.2000 Elsevier Science Ltd. All rights reserved.
Keywords: Allatostatin; Juvenile hormone biosynthesis; Diploptera punctata; Pseudopeptide mimetic analogues
1. Introduction
Cockroach allatostatins (ASTs) are a family of pep-tides, six to 18 amino acids in length, sharing the com-mon C-terminus Y/FXFGLamide. This pentapeptide sequence is the minimal sequence required for inhibition of juvenile hormone (JH) biosynthesis in vitro and is generally regarded as the core sequence responsible for direct receptor interaction. In many insect species, a reduction in endogenous titres of JH is critical for meta-morphosis from the nymph to the adult stage, whereas in adult females, oocyte growth and maturation show an
* Corresponding author. Tel.:+1-416-978-3517; fax:+ 1-416-978-3522.
E-mail address: [email protected] (S.S. Tobe).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 4 1 - 2
Dip-ASTs are released directly at the corpora allata (CA) and/or indirectly by release from the corpora car-diaca (Stay et al., 1992), midgut endocrine cells (Yu et al., 1995) or potentially from haemocytes (Skinner et al., 1997), travelling by way of the haemolymph to the CA or other targets. In vivo, ASTs in the haemolymph may inhibit JH biosynthesis by the CA. However, AST bioac-tivity can be reduced and ultimately terminated through catabolism by soluble haemolymph enzymes and by membrane-bound proteolytic enzymes (Garside et al., 1997a,b). The observed rates of catabolism suggest that haemolymph ASTs may not be effective humoral inhibi-tors of JH biosynthesis.
The results of these metabolic studies led to the design of a series of Dip-AST-derived pseudopeptide mimetic analogues. Our approach to the design of Dip-AST ana-logues with reduced susceptibility to metabolic inacti-vation has been both to eliminate those amino acids that are not required for biological activity by replacement with non-peptide moieties and to increase the rigidity of the molecule (Nachman et al., 1998). Radiochemical assay for JH biosynthesis indicates that the activities of these analogues approach those of the native neuropep-tides (Nachman et al. 1997, 1999). Studies on the catab-olism of Dip-AST analogues showed that selected ana-logues had increased resistance to catabolism by enzymes in the haemolymph or on the surface of tissues (Garside et al., 1997c; Nachman et al., 1999). It is likely that the significant biological activity of these peptidomi-metics is attributable in part to their increased resistance to catabolism.
The effects of ASTs in vivo have been comparatively understudied. Woodhead et al. (1993) observed a sig-nificant reduction in both rates of JH biosynthesis and in length of basal oocytes with Dip-AST 7 but only a significant reduction in rates of JH biosynthesis, not in length of basal oocytes, following injection of Dip-AST 2. In virgin P. americana, Weaver et al. (1995) demon-strated that both Dip-AST 5 and 7 were effective in low-ering total body JH III levels 12 h post injection, but not after 2 or 24 h. Injections of Dip-AST 7 into mid-cycle mated females produced no apparent effect but injection of Dip-AST 5 did result in a substantial reduction of endogenous total body JH III levels. Lorenz et al. (1998) injected Grb-AST A1 or B1 into G. bimaculatus and observed reductions in a number of physiological para-meters, including ovarian ecdysteroid biosynthesis and haemolymph vitellogenin titres compared with Ringer-injected controls.
Piulachs et al. (1997) synthesized a methyleneamino and a ketomethylene AST analogue with the aim of increasing resistance to degradation of ASTs by haemo-lymph peptidases. They showed that both analogues were similarly active to the model peptides with respect to the inhibition of JH biosynthesis in vitro from CA of virgin B. germanica. The methyleneamino analogue was
less active as an inhibitor of vitellogenin production in vitro by the fat body of B. germanica, but was more active in vivo in terms of both inhibition of JH biosynth-esis and as an inhibitor of vitellogenin production by the fat body.
In this study, we investigated the effects of injection of Dip-AST or Dip-AST analogue on rates of JH biosynthesis by CA in vitro and on basal oocyte growth of mated female D. punctata. Disruption in the cycle of JH biosynthesis could inhibit production or release of vitellogenin by fat body or uptake of vitellogenin by oocytes. Alternatively, reduction in the growth of basal oocytes may inhibit production of JH, either neurally or by release of an unknown humoral factor(s).
2. Materials and methods
2.1. Synthesis of allatostatin analogues
The AST pseudopeptide mimetic analogues 396-1 (Ala–Arg–Pro–Tyr–Asn–Aic–Gly–Leu–NH2, Aic= 2-amino-indane-2-carboxyl-), 397-2 (Ala–Arg–Pro–Tyr–
Asn–Phe–Cpa–Leu–NH2, Cpa=cyclopropylAla-),
AST(b)φ2 (Hca–Asn–Phe–Cpa–Leu–NH2, Hca= hydroc-innamyl-) and 419 (Ala–Arg–Pro–Tyr–Asn–Aic–Cpa– Leu–NH2) were synthesized as previously described (Nachman et al. 1998, 1999).
2.2. Animals
Newly emerged mated females were isolated from the stock culture and kept at 27±1°C until dissection and experimental manipulations. The relative humidity was approximately 50±5% with a 12 h light/12 h dark cycle. Insects were reared on Purina Lab Chow and water ad libitum. Mating was confirmed by the presence of a sper-matophore. Day 4 mated females were the source of corpora allata for analysis of JH biosynthesis and for measurement of basal oocyte length.
2.3. Degradation assay
Haemolymph collection and assay were as previously described (Garside et al., 1997a).
Tissue collection, membrane preparations and assay were as previously described (Garside et al., 1997b).
2.4. Injections
communication). Assuming a haemolymph volume of 50µl for adult female D. punctata (Mundall et al., 1981), the initial concentration of the injected peptide or ana-logue in the haemolymph was approximately 100µM. Injections of 6 nmol (1 nmol/µl) were administered once daily, approximately 24 h apart on days 0–3 inclusive, using a 26 gauge 10µl Hamilton syringe. The needle was inserted into the membranous joint between the coxa and femur on the metathoracic leg. Control insects were similarly injected, but with 6µl of autoclaved, double distilled water. On day 4, approximately 18 h after day 3 injection, animals were sacrificed; JH biosynthesis by CA was measured in vitro by the radiochemical assay (Tobe and Clarke, 1985) and the length of basal oocytes was measured using an ocular micrometer. Each group of peptide-injected animals (4×6 nmol) was compared with a group of water-injected animals treated concur-rently.
2.5. Animal weight, feeding, excretion and soluble protein content of fat body
On days 1 to 4 (d1–4), animals were measured for food consumption, production of frass, and weight. Food pel-lets and animals were weighed prior to segregation in jars. Food consumption and production of frass were each approximated by weighing of food pellets and frass approximately 18 h following the previous day’s injec-tion on each of d1–4. Food consumpinjec-tion was determined by the subtraction of weight of food pellet on dX from
weight on dX21 for each day. Animal weight was meas-ured approximately 18 h following the previous days injection on each of days 1 to 4. Percentage weight gain=[(Weightd42Weightd0)/Weightd0]×100%. Soluble protein content of fat body from day 4 mated females was also determined in these same animals by transfer of abdominal fat body from each animal to a pre-weighed vessel. Excess fluid was removed by blotting with filter paper and the wet weight measured. The fat body was homogenized in 1 N NaOH and centrifuged at 4000g for 10 min. The quantity of soluble protein was determined by the Bradford (1976) method using bovine serum albumin as standard. Data are presented as µg protein/mg fat body.
2.6. Radiochemical assay
Rates of JH release were determined by the in vitro radiochemical method of Feyereisen and Tobe (1981) and Tobe and Clarke (1985). The incorporation of l-[ 14C-S-methyl]-methionine (50µM, specific radioactivity
1.48–2.03 GBq/mmol; Amersham) into JH III by pairs of CA incubated in 100µl TC 199 (GIBCO; 1.3 mM Ca2+, 2% Ficoll, methionine-free) was used to quantify JH release. Animals were anaesthetized on ice prior to dissection. Corpora allata were dissected directly into
non-radioactive medium. Individual pairs of CA were transferred from non-radioactive medium to radioactive medium and were incubated for 3 h. The amount of inhi-bition was expressed as the percentage reduction from the untreated rate: [12(treated rate/untreated rate)]×100%.
2.7. Statistics
Data were analysed using a one-way analysis of vari-ance (ANOVA) with a Dunnett’s multiple comparison test as the post hoc determination of significance and a Student’s t-test using Prism Graph Pad. Variability is reported as ±standard error of the mean (SEM).
3. Results
To determine the least injurious protocol for injection of animals, preliminary experiments were performed on animals that were chilled at 4°C, and on control animals that had not been chilled prior to injection. It was found that chilling substantially reduced the rates of JH biosynthesis in vitro and also reduced the length of basal oocytes compared with controls (data not shown). As a result, in this study, non-chilled animals were used exclusively.
For this study, we chose AST 5 and 7, and Dip-AST analogues 397-2, 396-1, 419 and Dip-AST(b)φ2 to test in vivo. Dip-AST 7 was used because it is one of the most potent ASTs with respect to the inhibition of JH biosynthesis (Tobe et al., 2000) and permits a direct comparison to the studies of Woodhead et al. (1993). Dip-AST 5 is also a potent inhibitor of JH biosynthesis, and shows a prolonged half-life in haemolymph com-pared with other Dip-ASTs (.15×) (Garside et al., 1997a). Analogues 397-2, 396-1 and 419 were chosen because they showed significant inhibition of JH biosynthesis in vitro (Garside et al., 1997c; Garside, unpublished; Nachman et al. 1997, 1999). Analogue AST(b)φ2 was chosen because it showed significant inhibition of JH biosynthesis and also because it exhib-ited high resistance to both haemolymph and membrane-bound peptidases (Garside et al., 1997c; Nachman et al. 1997, 1999). Control animals were injected with the same volume of solvent or the same volume and concen-tration of Dip-AST 7(1–7) N-terminal heptapeptide. This fragment does not possess the C-terminal active core of the Dip-ASTs and lacks JH inhibitory activity in vitro (Garside, unpublished).
3.1. Effects on JH biosynthesis
Fig. 1. Rates of JH biosynthesis of corpora allata from (A) uninjected animals; and (B) animals injected with water (control) or selected Dip-ASTs or Dip-AST analogues. The identity of the compounds represented in the bars is indicated in the legend. Measurements were made on day 4. Each bar represents the mean±SEM for the number of individual measurements indicated at the top of error bars. Asterisks indicate significant differences between peptide- and water-injected groups of animals as determined by a Dunnett’s multiple comparison test following one-way ANOVA: *, 0.01,P,0.05; **, 0.001,P,0.01. Significance determined by a Student’s t-test were: AST 7(1–7), not significant (NS); Dip-AST 7 and analogues 396-1 and 419,,0.05; Dip-AST 5 and analogues 397-2 and AST(b)φ2,,0.001.
by CA from animals injected with water, Dip-AST or Dip-AST analogue. Untreated animals showed rates of JH biosynthesis similar to those of water-injected con-trols (104.6±6.8 pmol h21 per pair versus 101.9±6.2 pmol h21per pair, respectively). Thus, a 6µl injection of water did not adversely affect the rate of JH biosynthesis. In this experiment we used water-injected animals as controls for statistical analyses. Injection of Dip-AST 7(1–7) did not significantly alter rates of JH biosynthesis whereas injection of AST 7 or Dip-AST 5 did result in a significant (P,0.05 and P,0.01, respectively) reduction in the rate of JH biosynthesis. Injection of pseudopeptide analogues 396-1 and 419 also resulted in reductions in the rates of JH biosynthesis (25.0% and 29.6%, respectively) but these values were not significantly different from control animals. How-ever, analogues 397-2 and AST(b)φ2 decreased the rates of JH biosynthesis significantly (P,0.01), with AST(b)φ2 exhibiting an average level of inhibition of greater than 50%.
3.2. Effects on length of basal oocytes
Fig. 2(A) shows the mean length of basal oocytes from untreated day 4 mated female D. punctata. Fig. 2(B) shows the mean length of basal oocytes from ani-mals injected with water, Dip-AST or Dip-AST ana-logue. The mean length of basal oocytes from untreated animals was significantly greater than that of water-injected controls (1.43±0.02 mm versus 1.21±0.03 mm) (P,0.05). For this reason, we used water-injected ani-mals as controls for statistical analyses. Injection of
Dip-AST 7(1–7) or Dip-Dip-AST 7 did not result in a significant reduction in the length of basal oocytes. Injection of Dip-AST 5 or analogue 419 did result in a significant reduction in basal oocyte growth during the first four days of adult life (P,0.01 and P,0.05, respectively). Injection of analogues 396-1, 397-2 and AST(b)φ2 also resulted in a highly significant reduction in the growth of basal oocytes in early adult life (P,0.01). Specifically, injection of analogues AST(b)φ2 and 397-2 resulted in a greater than 25% reduction in mean length of basal oocytes.
3.3. Effects on animal weight, feeding, excretion and soluble protein content of fat body
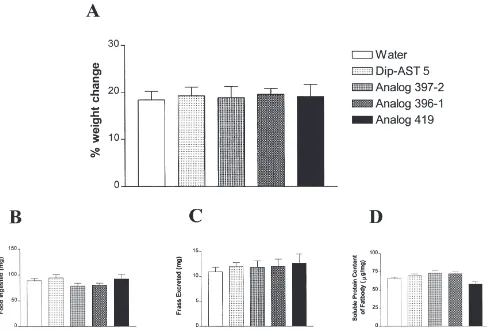
Fig. 3 displays other physiological responses to injec-tion of Dip-AST 5 or Dip-AST analogue. Once again, injection of water served as the control to which all other results were compared. Injection of these compounds had no significant effect on weight gain, consumption of food, excretion of frass, or on the soluble protein content of fat body during the experimental period.
4. Discussion
Fig. 2. Length of basal oocytes from (A) uninjected animals; and (B) animals injected with water (control) or selected Dip-ASTs or Dip-AST analogues. The identity of the compounds represented in the bars is indicated in the legend. Measurements were made on day 4. Each bar represents the mean±SEM for the number of individual measurements indicated at the top of error bars. Asterisks indicate significant differences between peptide- and water-injected groups of animals as determined by a Dunnett’s multiple comparison test following one-way ANOVA: *, 0.01,P,0.05; **, 0.001,P,0.01. Significance determined by a Student’s t-test were: Dip-AST 7(1–7), NS; Dip-AST 7 and analogue 419,,0.05; Dip-AST 5 and analogues 397-2, 396-1 and AST(b)φ2,,0.001.
is potentially one of the most important mechanisms reg-ulating peptide action. We have previously shown that Dip-ASTs are cleaved and ultimately inactivated by enzymes in both haemolymph and bound to tissue mem-brane preparations (Garside et al., 1997a,b). For these reasons, we designed a series of AST pseudopeptide ana-logues.
In the present experiments, we have shown that injec-tion of specific Dip-ASTs or Dip-AST analogues results in a significant reduction in the rate of JH biosynthesis and/or a significant reduction in basal oocyte growth. Concurrent with these experiments, we also investigated the effect of injection of selected compounds on weight gain, feeding, excretion, and soluble protein content of fat body. We observed no significant change in any of these physiological parameters, indicating that gut motility, and protein absorption and utilization, were not affected by injection of these compounds. These obser-vations support the suggestion that Dip-ASTs and their analogues directly affect JH biosynthesis by the CA and inhibit the growth of basal oocytes, and are not toxic to the insect.
4.1. Native ASTs
Dip-AST 7 is a potent inhibitor of JH biosynthesis in vitro. However, it is subject to degradation by haemo-lymph peptidases, (T1/2=22 min; Garside et al., 1997a). In the present experiments, Dip-AST 7 significantly inhibited the rate of JH biosynthesis but did not signifi-cantly inhibit the growth of basal oocytes, whereas Woodhead et al. (1993) found that Dip-AST 7 signifi-cantly inhibited both parameters. This may be attribu-table to differences in experimental protocol (Woodhead et al. injected two times per day, thereby causing more mechanical trauma, whereas we injected only once per day). Tests for statistical significance also differed between the two studies (Dunnett’s multiple comparison test following ANOVA versus Student’s t-test used by Woodhead et al., 1993).
Dip-AST 5 exhibits an extended half-life
(T1/2=153 min) in the haemolymph of D. punctata, sug-gesting that it plays an important humoral role in this species (Garside et al., 1997a). Our results demonstrate that Dip-AST 5 significantly inhibited both the biosynth-esis of JH and the growth of basal oocytes (see Figs. 1 and 2). Thus, the haemolymph titre of Dip-AST 5 may play a role in the regulation of JH biosynthesis at specific stages during the life cycle of the cockroach.
4.2. AST pseudopeptide mimetic analogues
The bioactivity in vitro of the neuropeptide analogues approached and, in the case of analogue 396-1, almost matched that of the native ASTs (Garside et al., 1997c; Nachman et al. 1997, 1999). These studies also revealed
that several analogues were resistant to enzymatic degra-dation, most notably AST(b)φ2, which was highly resist-ant to catabolic enzymes in the haemolymph and mem-brane preparations.
Injection of analogue 397-2 significantly inhibited both the rate of JH biosynthesis and the length of basal oocytes. Surprisingly, analogues 396-1 and 419 had no significant effect on the rate of JH biosynthesis, but did significantly inhibit the growth of basal oocytes (see Fig. 2). This is surprising, because analogue 419 shares a common substitution (Cpa) with analogue 397-2. Analy-sis of haemolymph peptidase hydrolyAnaly-sis revealed that analogues 397-2 and 396-1 were targeted outside the active core region. Membrane-bound peptidases also primarily cleaved these analogues outside the active core. However, in membrane preparations, 396-1 and 397-2 were secondarily cleaved at positions not targeted in native peptides (Nachman et al., 1997). These result-ant cleavages are in the active core of the ASTs and would result in a loss of biological activity. It is possible that the fragment generated from the primary cleavage of 397-2 has a longer half-life than that of 396-1. This may account for the increased potency in vivo of 397-2. In addition, although not shown here, the half-life of analogue 397-2 is three times greater than those of ana-logue 396-1 and Dip-AST 5 in incubations with brain membrane preparations. This resistance to membrane-bound catabolic enzymes may provide an explanation for the potency in vivo exhibited by 397-2, even though its
IC50in vitro is two orders of magnitude higher than that of 396-1. Although these studies have not yet been car-ried out on analogue 419, it can be predicted that the primary cleavages of this analogue would also be outside the active core, since it possesses both the Aic from 396-1 and the Cpa from 397-2.
AST(b)φ2 exhibited high resistance to degradation by enzymes in haemolymph and by enzymes of crude mem-brane preparations of brain and midgut (Garside et al., 1997c; Nachman et al., 1999), and retained significant biological activity in vitro. Not surprisingly, this ana-logue significantly (P,0.01) inhibited both the biosynth-esis of JH and the growth of basal oocytes. In fact, basal oocyte length in AST(b)φ2 injected animals on day 4 was similar to untreated mated female D. punctata on day 2 (0.88 mm), demonstrating that this analogue delayed vitellogenesis by approximately 36 h. The effec-tiveness in vivo of AST(b)φ2 may be a consequence of its resistance to peptidase degradation.
against haemolymph peptidase attack, but not against membrane-bound peptidase attack. However, haemo-lymph hydrolysis leads to fragments that retain biologi-cal activity (Garside et al., 1997a), whereas membrane-bound peptidase hydrolysis inactivates the peptide (Garside et al., 1997b). Our analogues are protected against attack by membrane-bound peptidases that cleave Dip-AST 5 in the active core (Garside et al., 1997b). For these reasons, we believe that our analogues represent ideal tools for investigating effects in vivo of injected AST-like compounds.
In this study, we investigated the effects in vivo of Dip-AST and Dip-AST pseudopeptide analogues on rates of JH biosynthesis and basal oocyte growth. How-ever, basal oocyte growth may be a more meaningful assay of the effectiveness of an injected AST or AST analogue since it is a cumulative measure of the effect of the injections on JH biosynthesis and titre, and on vitellogenin release and uptake. In contrast, the measure-ment of JH biosynthesis is made at a single point in time, from a gland removed from its internal milieu, approxi-mately 18 h after the last injection.
In vivo, JH biosynthesis, basal oocyte growth and indeed AST action can be affected by other tissues and/or factors involved in the mediation of the response. For example, hormone titres are regulated by a variety of factors including their synthesis, degradative metab-olism, sequestration and excretion (Hammock, 1985; Stay et al., 1994). One of the most important factors contributing to the observed results in vivo may be the contribution of feedback loops incorporating nervous connections, humoral factors (e.g., ASTs, ecdysteroids and JHs) and their titres, CA sensitivity, vitellogenin synthesis and release from the fat body, and its uptake by the ovary. The interpretation of results obtained from studies in vivo are confounded by these factors and, therefore, our results may only be attributable in part to the direct effects of the injected compound.
ASTs could also inhibit the glycosylation of vitellog-enin in the fat body, which in turn may reduce the circul-ating vitellogenin titre, thereby reducing the uptake of vitellogenin by the basal oocytes. Martı´n et al. (1996) showed that AST impaired vitellogenin release from fat body in vitro and that this effect was mediated by the inhibition of vitellogenin glycosylation in periovarian fat body of B. germanica. Lorenz et al. (1998) have shown that injection of Grb-AST A1 into G. bimaculatus reduces the biosynthesis of ovarian ecdysteroids which may in turn influence JH biosynthesis (Hoffmann and Gerstenlauer, 1997). However, this effect was not sig-nificant, nor was this response manifest in vitro.
Different ASTs may have different effects at different stages of development. Indeed, our study shows that ASTs differ in their potencies with respect to inhibition of JH biosynthesis and of basal oocyte growth. Also, cocktails of ASTs may be involved in the response in
vivo. At present, we have no evidence for differential processing of the AST precursor, although this may be a means by which the cockroach can deliver specific active ASTs to specific target tissues.
An impediment to the successful use of AST ana-logues in the field is the problem of delivery of the com-pounds to the animal. We have produced potent ana-logues resistant to catabolism, but these compounds do not penetrate the cuticle or gut wall. Addition of a hydro-phobic moiety to an active portion of these analogues may provide amphiphilic compounds capable of pen-etrating the cuticle while still retaining significant bio-logical activity.
Acknowledgements
Financial support was provided by a Karlson Foun-dation Fellowship to C.S.G. and a Natural Sciences and Engineering Research Council of Canada operating grant to S.S.T. We would like to thank Sylvia Ley for techni-cal assistance.
References
Belle´s, X., Maestro, J.L., Piulachs, M.D., Johnsen, A.H., Duve, H., Thorpe, A., 1994. Allatostatic neuropeptides from the cockroach
Blattella germanica (L) (Dictyoptera, Blattellidae). Identification,
immunolocalization and activity. Regul. Pept. 53, 237–247. Bradford, M.M., 1976. A rapid and sensitive method for the
quantit-ation of microgram quantities of protein utilizing the principal of protein–dye binding. Anal. Biochem. 72, 248–254.
Feyereisen, R., Tobe, S.S., 1981. A rapid partition assay for routine analysis of juvenile hormone release in insect corpora allata. Anal. Biochem. 111, 372–375.
Fuse´, M., Zhang, J.R., Partridge, E., Nachman, R.J., Orchard, I., Bendena, W.G., Tobe, S.S., 1999. Effects of an allatostatin and a myosuppressin on midgut carbohydrate enzyme activity in the cockroach Diploptera punctata. Peptides 20, 1285–1293. Garside, C.S., Hayes, T.K., Tobe, S.S., 1997a. Degradation of
Dip-allatostatins by haemolymph from the cockroach, Diploptera
punctata. Peptides 18, 17–25.
Garside, C.S., Hayes, T.K., Tobe, S.S., 1997b. Inactivation of Dip-allatostatin 5 by membrane preparations from the cockroach
Diploptera punctata. Gen. Comp. Endocrinol. 108, 258–270.
Garside, C.S., Nachman, R.J., Tobe, S.S., 1997c. Catabolism of insect neuropeptides: allatostatins as models for peptidomimetic design. In: Kawashima, S., Kikuyama, S. (Eds.), Advances in Comparative Endocrinology. Monduzzi Editore, Yokohama, pp. 1353–1359. Hammock, B.D., 1985. Regulation of juvenile hormone titer.
Degra-dation. In: Kerkut, G.A., Gilbert, L.I. (Eds.). Comprehensive Insect Physiology, Biochemistry and Pharmacology, vol. 7. Pergamon Press, Oxford, pp. 431–472.
Hoffmann, K.H., Gerstenlauer, B., 1997. Effects of ovariectomy and allatectomy on ecdysteroid synthesis and ecdysteroid titers during larval–adult development of Gryllus bimaculatus de Geer (Ensifera, Gryllidae). Arch. Insect Biochem. Physiol. 35, 149–158. Lange, A.B., Chan, K.K., Stay, B., 1993. Effect of allatostatin and
Lange, A.B., Bendena, W.G., Tobe, S.S., 1995. The effect of the thir-teen Dip-ASTs on myogenic and induced contractions of the cock-roach (Diploptera punctata) hindgut. J. Insect Physiol. 41, 581– 588.
Lorenz, M.W., Kellner, R., Hoffmann, K.H., 1995. Identification of two allatostatins from the cricket, Gryllus bimaculatus de Geer (Ensifera, Gryllidae): additional members of a family of neuropep-tides inhibiting juvenile hormone biosynthesis. Regul. Pept. 57, 227–236.
Lorenz, M.W., Lorenz, J.I., Treiblmayr, K., Hoffmann, K.-H., 1998. In vivo effects of allatostatins in crickets, Gryllus bimaculatus (Ensifera: Gryllidae). Arch. Insect Biochem. Physiol. 38, 32–43. Martı´n, D., Piulachs, M.D., Belle´s, X., 1996. Inhibition of vitellogenin
production by allatostatin in the German cockroach. Mol. Cell. Endocrinol. 121, 191–196.
Mundall, E.C., Tobe, S.S., Stay, B., 1981. Vitellogenin fluctuations in haemolymph and fat body and dynamics of uptake into oocytes during the reproductive cycle of Diploptera punctata. J. Insect Phy-siol. 27, 821–827.
Nachman, R.J., Moyna, G., Williams, H.J., Garside, C.S., Tobe, S.S., 1997. Active conformation and peptidase resistance of confor-mationally restricted analogues of the insect allatostatin neuropep-tide family. In: Kawashima, S., Kikuyama, S. (Eds.), Advances in Comparative Endocrinology. Monduzzi Editore, Yokohama, pp. 1353–1359.
Nachman, R.J., Moyna, G., Williams, H.J., Tobe, S.S., Scott, A., 1998. Synthesis, biological activity, and conformational studies of insect allatostatin neuropeptide analogs incorporating turn promoting moi-eties. Bioorganic Med. Chem. 6, 1379–1388.
Nachman, R.J., Garside, C.S., Tobe, S.S., 1999. Haemolymph and tissue-bound peptidase resistant analogs of the insect allatostatins. Peptides 20, 23–29.
Piulachs, M., Vilaplana, L., Bartolome´, J., Carren˜o, C., Martı´n, D., Gonza´les-Mun˜iz, R., Herranz, R., Garcı´a-Lo´pez, M., Andreu, D., Belle´s, X., 1997. Ketomethylene and methyleneamino pseudopep-tide analogues of insect allatostatins inhibit juvenile hormone and vitellogenin production in the cockroach Blattella germanica. Insect Biochem. Mol. Biol. 27, 851–858.
Pratt, G.E., Farnsworth, D.E., Siegel, N.R., Fok, K.F., Feyereisen, R., 1989. Identification of an allatostatin from adult Diploptera
punctata. Biochem. Biophys. Res. Commun. 163, 1243–1247.
Pratt, G.E., Farnsworth, D.E., Fok, K.F., Siegel, N.R., McCormack, A.L., Shabanowitz, J., Hunt, D.F., Feyereisen, R., 1991. Identity of a second type of allatostatin from cockroach brains: an octadeca-peptide amide with tyrosine-rich address sequence. Proc. Nat. Acad. Sci., USA 88, 2412–2416.
Predel, R., Kellner, R., Rapus, J., Ga¨de, G., 1999. Allatostatins from the retrocerebral complex and antennal pulsatile organ of the amer-ican cockroach: structural elucidation aided by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. Regul. Pept. 82, 81–89.
Skinner, J.R., Fairbairn, S.E., Woodhead, A.P., Bendena, W.G., Stay, B., 1997. Allatostatin in hemocytes of the cockroach Diploptera
punctata. Cell Tissue Res. 290, 119–128.
Stay, B., Chan, K.K., Woodhead, A.P., 1992. Allatostatin-immunore-active neurons projecting to the corpora allata of adult Diploptera
punctata. Cell Tissue Res. 270, 15–23.
Stay, B., Sereg Bachmann, J.A., Stoltzman, C.A., Fairbairn, S.E., Yu, C.G., Tobe, S.S., 1994. Factors affecting allatostatin release in a cockroach (Diploptera punctata): nerve section, juvenile hormone analogue and ovary. J. Insect Physiol. 40, 365–372.
Tobe, S.S., Clarke, N., 1985. The effect ofl-methionine concentration on juvenile hormone biosynthesis by corpora allata of the cock-roach Diploptera punctata. Insect Biochem. 15, 175–179. Tobe, S.S., Zhang, J.R., Bowser, P.R.F., Donly, B.C., Bendena, W.G.,
2000. Biological activities of the allatostatin family of peptides in the cockroach, Diploptera punctata and potential interactions with receptors. J. Insect Physiol. 46, 231–242.
Weaver, R.J., Freeman, Z.A., Pickering, M.G., Edwards, J.P., 1994. Identification of two allatostatins from the CNS of the cockroach
Periplaneta americana: novel members of a family of neuropeptide
inhibitors of insect juvenile hormone biosynthesis. Comp. Biochem. Physiol. 107C, 119–127.
Weaver, R.J., Paterson, Z.A., Short, J.E., Edwards, J.P., 1995. Effects of Diploptera punctata allatostatins on juvenile hormone biosynth-esis and endogenous juvenile hormone III levels in virgin and mated female Periplaneta americana. J. Insect Physiol. 41, 117– 125.
Woodhead, A.P., Stay, B., Seidel, S.L., Khan, M.A., Tobe, S.S., 1989. Primary structure of four allatostatins: neuropeptide inhibitors of juvenile hormone synthesis. Proc. Nat. Acad. Sci., USA 86, 5997–6001.
Woodhead, A.P., Asano, W.Y., Stay, B., 1993. Allatostatins in the haemolymph of Diploptera punctata and their effect in vivo. J. Insect Physiol. 39, 1001–1005.
Woodhead, A.P., Khan, M.A., Stay, B., Tobe, S.S., 1994. Two new allatostatins from the brains of Diploptera punctata. Insect Biochem. Mol. Biol. 24, 257–263.