David Publishing Company www.davidpublishing.com P u b l i s h i n g Dav i d
Journal of Life Sciences
Publication Information
Journal of Life Sciences is published monthly in hard copy (ISSN 1934-7391) and online (ISSN 1934-7405) by David Publishing Company located at 1840 Industrial Drive, Suite 160, Libertyville, Illinois 60048, USA.
Aims and Scope
Journal of Life Sciences, a monthly professional academic journal, covers all sorts of researches on molecular biology, microbiology, botany, zoology, genetics, bioengineering, ecology, cytobiology, biochemistry, and biophysics, as well as other issues related to life sciences.
Editorial Board Members
Dr. Stefan Hershberger (USA), Dr. Suiyun Chen (China), Dr. Farzana Perveen (Pakistan), Dr. Francisco Torrens (Spain), Dr. Filipa João (Portugal), Dr. Masahiro Yoshida (Japan), Dr. Reyhan Erdogan (Turkey), Dr. Grzegorz Żurek (Poland), Dr. Ali Izadpanah (Canada), Dr. Barbara Wiewióra (Poland), Dr. Valery Lyubimov (Russia), Dr. Amanda de Moraes Narcizo (Brasil), Dr. Marinus Frederik Willem te Pas (The Netherlands), Dr. Anthony Luke Byrne (Australia), Dr. Xingjun Li (China), Dr. Stefania Staibano (Italy), Dr. Wenle Xia (USA), Hamed Khalilvandi-Behroozyar (Iran).
Manuscripts and correspondence are invited for publication. You can submit your papers via Web Submission, or E-mail to [email protected] or [email protected]. Submission guidelines and Web Submission system are available at http://www.davidpublishing.com.
Editorial Office
1840 Industrial Drive, Suite 160, Libertyville, Illinois 60048 Tel: 1-847-281-9826, Fax: 1-847-281-9855
E-mail:[email protected], [email protected]
Copyright©2011 by David Publishing Company and individual contributors. All rights reserved. David Publishing Company holds the exclusive copyright of all the contents of this journal. In accordance with the international convention, no part of this journal may be reproduced or transmitted by any media or publishing organs (including various websites) without the written permission of the copyright holder. Otherwise, any conduct would be considered as the violation of the copyright. The contents of this journal are available for any citation. However, all the citations should be clearly indicated with the title of this journal, serial number and the name of the author.
Abstracted / Indexed in
Database of EBSCO, Massachusetts, USA Cambridge Scientific Abstracts (CSA), USA
Chinese Database of CEPS, American Federal Computer Library Center (OCLC), USA Ulrich’s Periodicals Directory, USA
Chinese Scientific Journals Database, VIP Corporation, Chongqing, China Summon Serials Solutions
Subscription Information
Price (per year): Print $420, Online $300, Print and Online $560
David Publishing Company
1840 Industrial Drive, Suite 160, Libertyville, Illinois 60048 Tel: 1-847-281-9826, Fax: 1-847-281-9855
E-mail: [email protected]
Dav id Publishing Company
w ww.davidpublis hing.com
Pu b li sh i ng
Dav i d
J LS
Journal of Life Sciences
Volume 5, Number 7, July 2011 (Serial Number 39)
Contents
Research Papers
483 Differential Expression of Wnts, β-catenin and E-cadherin in hEFs and Normal, Abnormal Karyotype hES Cells during Culture in vitro
Xueqin Zheng, Zhen Xiang, Xianlei Li, Wenling Lu, Li Tan, Qingguo Luo, Changqing He, Ye Yu, Yi Yao, Ying Li, Huaijiang Li and Yang Xiang
488 Identification, Cloning and Characterization of Dictyoglomus Turgidum CelA, an Endoglucanase with Cellulase and Mannanase Activity
Phillip J. Brumm, Spencer Hermanson, Joshua Luedtke and David A. Mead
497 Evaluation of Barley Genotypes for Resistance to Pyrenophora Teres Using Molecular Markers
Leona Leisova-Svobodova, Lenka Stemberková, Martina Hanusová and Ladislav Kučera
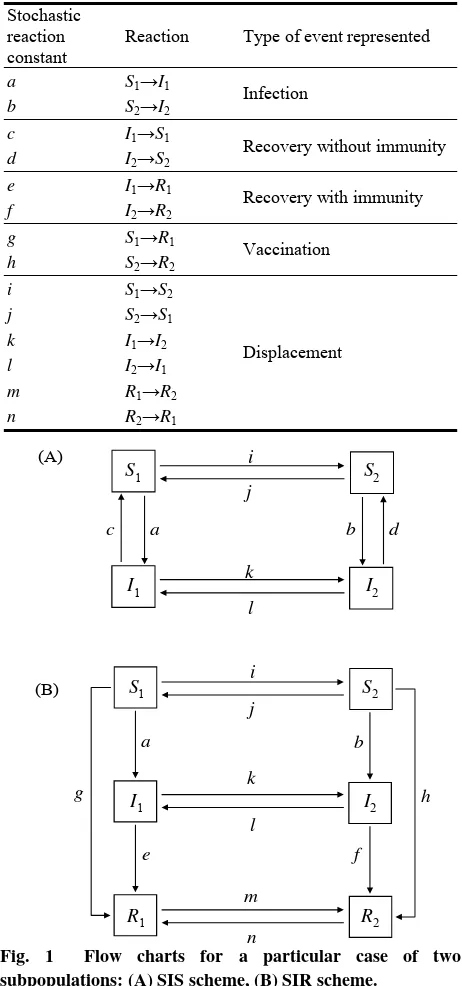
503 Dynamics and Control of Infectious Diseases in Stochastic Metapopulation Models
Ariel Félix Gualtieri and Juan Pedro Hecht
509 The Research on Phosphate Mobilizing Bacteria from Soils of Southern Ukraine
Ludmila Chaikovska
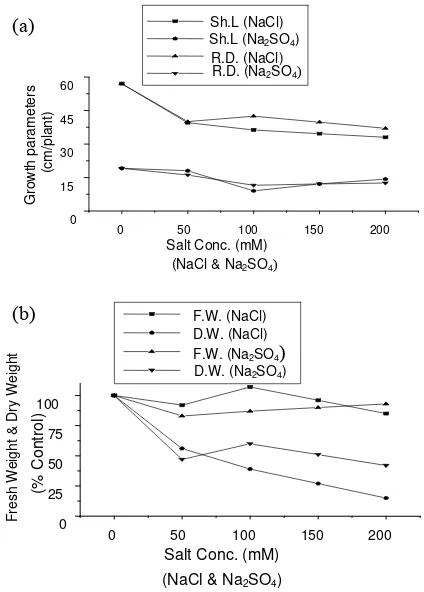
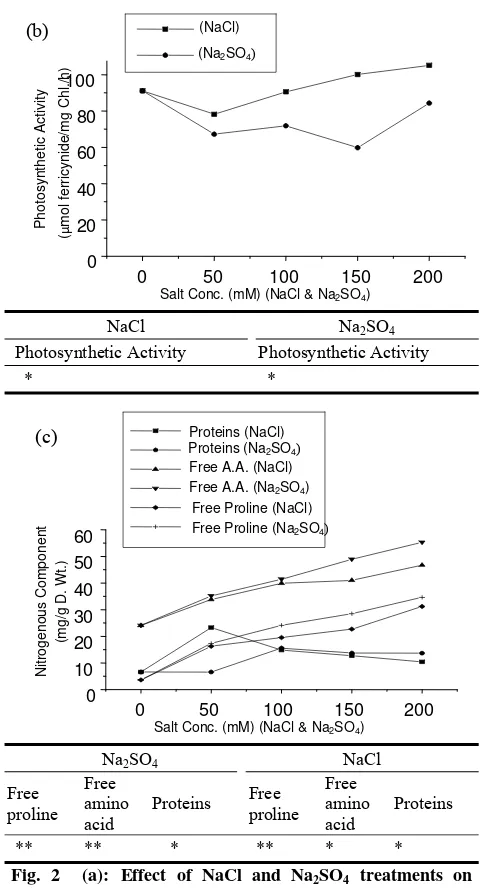
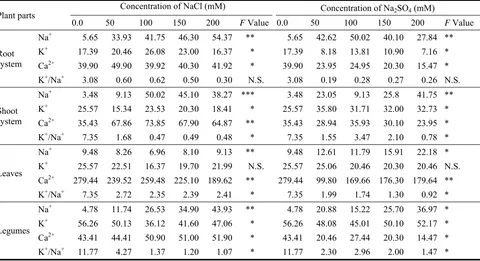
513 Influence of NaCl and Na2SO4 Treatments on Growth Development of Broad Bean (Vicia Faba, L.) Plant
Hameda El Sayed Ahmed El Sayed
524 Obsolete Pesticides and Phytoremediation of Polluted Soil in Kazakhstan
Asil Nurzhanova, Kabyl Zhambakin, Issbacar Rakhimbayev, Anatoly Sedlovskiy and Sergey Kalugin
536 Effect of Inorganic and Organic Based Fertilizers on Growth Performance of Tea and Cost Implications in Kusuku, Nigeria
Rotimi Rufus Ipinmoroti, Gerald Oaikhena Iremiren, Olayiwola Olubamiwa, Akanbi Olutayo Fademi and Emmanuel Ogieriakhi Aigbekaen
541 The Effectiveness of Watermelon Endocarp Extract in Inhibiting Lipase Activity Relative to the Hypolipidemic Drugs
546 A New Record of Doria’s Comb Fingered Gecko, Stenodactylus Doriae, (Blanford, 1874), (Reptilia: Gekkonidae) from Southeastern of Iran, Sistan & Baluchistan Province
Nastaran Heidari, Nasrullah Rastegar-Pouyani and Hiva Faizi
549 Does Procambarus Clarkii (Girard, 1852) Represent a Threat for Estuarine Brackish Ecosystems of Northeastern Adriatic Coast (Italy)?
Sandra Casellato and Luciano Masiero
555 Metabolizable Energy and Amino Acid Bioavailability of Field Pea Seeds in Broilers Diets
Vassilios Dotas, Asterios Hatzipanagiotou and Konstantinos Papanikolaou
562 Chemical Composition of Meat in Castrated Male Brahman Cattle in Venezuela
José A. Miguel, Jesús Ciria, Begoña Asenjo, Hector Pargas and David Colmenarez
569 Alternative Distributions to Estimate Usual Intake of Nutrients for Groups
José Eduardo Corrente, Juliana M. Morimoto, Dirce Maria Lobo Marchioni and Regina Mara Fisberg
575 Computer Supported Sensory Profiling Analysis of Three Agaricus Cultivars
Journal of Life Sciences 5 (2011) 483-487
Differential Expression of
Wnts
,
β
-catenin
and
E-cadherin
in hEFs and Normal, Abnormal Karyotype
hES Cells during Culture
in vitro
Xueqin Zheng, Zhen Xiang, Xianlei Li, Wenling Lu, Li Tan, Qingguo Luo, Changqing He, Ye Yu, Yi Yao, Ying Li, Huaijiang Li and Yang Xiang
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang 414000, China
Received: March 07, 2011 / Accepted: March 24, 2011 / Published: July 30, 2011.
Abstract: Human embryonic fibroblasts (hEFs) can well maintain the pluripotency in human embryonic stem cells (hESs). However, recent research and reports indicated that a few of hES cell lines acquired genomic alteration during long-term culture of hES cells in
vitro. This will directly restrict the therapy use of hES cells. Wnts are secreted lipid-modified signaling proteins that influence multiple
processes ranging from cell proliferation to stem cell loss. Activation of Wnt signaling in many tissues has also been associated with cancer. Unchecked Wnt signaling and loss of cadherin expression can promote tumorigenesis. In this study, we found the caryotype of one hES cell line chHES-3 changed with duplication of 1p32-1p36 area after 34 passages. The results of RT-PCR indicated Wnt7a was expressed in hEFs after culture normal karyotype hES cells, but not expressed in control and abnormal karyotype hES cells. Wnt3 was expressed in hEFs after culture abnormal karyotype hES cells, not expressed in control and normal karyotype hES cells. Wnt3, Wnt9a
and Wnt10b were detected weakly expression in normal hES cells, but higher in abnormal hES cells. At the same time, Wnt3a, Wnt4,
Wnt5b, Wnt7a, Wnt8b and Wnt11 were expressed and E-cadherin was not tested in abnormal hES cells compared with normal hES cells.
All that indicated Wnt7a was need for culture normal karyotype hES cells and Wnt3 was need for culture abnormal karyotype hES cells on hEFs. Wnt3, Wnt9a and Wnt10b high expression in hES cells and absence of E-cadherin may cause hES cells karyotype change.
Key words: hEFs, hES cell karyotype, differential expression, Wnts, β-catenin, E-cadherin.
1. Introduction
Human embryonic stem cells are derived from inner cell mass of human blastocyst and will be the unlimited cell source for future cell therapy, contributing to their characteristics of pluripotency, capability of differentiation to almost any type of cells [1]. At present a lot of inactive mouse embryonic fibroblasts (mEFs) and human embryonic fibroblasts are required to maintain and support the hES cell growth in an undifferentiated state [2-4]. hES cells have held great promise for clinical use as a major resource in future cell and tissue replacement therapy, but concerns are always kept on the safety of hES cells as long term
Corresponding author: Yang Xiang, Prof., research field: molecular and cell biology. E-mail: [email protected].
culture of any indefinite cell lines will lead to gene mutation and chromosome aberration which may confer the cells a malignant phenotype [5]. More hES cell lines could well maintain a normal karyotype during prolonged culture, but,a few of them did acquire chromosomal changes [6]. In our laboratory, we found the caryotype of one hES cell line chHES-3 was changed with duplication of 1p32-1p36 area after 34 passages.
cell factor/lymphoid enhancer factor TCF/LEF in the Wnt pathway [9] and a structural adaptor protein linking cadherins to the actin cytoskeleton in cell-cell adhesion[10]. Wnts influence multiple processes in animal development [8]. Nineteen Wnt genes exist in mammalian genomes, and the diversity of their functions is exemplified by mutations that lead to developmental abnormalities ranging from stem cell loss to kidney and reproductive tract defects[9]. In many tissues, activation of Wnt signaling has also been associated with cancer [11]. Recent studies have shown that both Wnt signaling and cadherin-mediated cell-cell adhesion are important in the organization and maintenance of stem cells [12]. Alterations in cell fate, adhesion, and migration are characteristics of cancer in which cells ignore normal regulatory cues from their environment. Unchecked Wnt signaling [13] and/or the loss of cell-cell adhesion [14-15]are involved in cancer induction and progression. Loss of cadherin expression can also promote tumorigenesis [14-15].
In our study, we used RT-PCR to analyze the differential expression of Wnts, β-catenin and
E-cadherin in hEFs and normal and abnormal caryotype hES cells during culture in vitro, so as to filter the candidate genes that may maintain hES cells normal karyotype or urge hES cells karyotypic change. Our study suggested that Wnt7a was needed for culture normal karyotype hES cells and Wnt3 was needed for culture abnormal karyotype hES cells on hEFs. High expression of Wnt3, Wnt9a and Wnt10b in hES cells and absence expression of E-cadherin may cause hES cells karyotype change.
2. Materials and Methods
2.1 hEFs Isolation and Culture
hEFs as feeder cells derived from two month abortion fetus musculature were harvested hES cells in our laboratory according to the method described in Ref. [16]. The experiment was conducted according to the Guide for the Care and Use of Laboratory Animals, enunciated by the Ministry of Science and Technology
of the People’s Republic of China. The fibroblasts were routinely expanded in DMEM (Gibco Invitrogen, USA) supplemented with 10% Fetal calf serum (Haita). Seventy to eighty percent of confluent cells were rendered mitotically inactive by being resuspended in medium and treated them with 33G of gamma irradiation. Inactive cells were related with a dilution of 8×105/mL into pre-gelled plates. The batch of hEFs which could support the growth of hES cells were subjected to proceed to next steps. hEFs in the control group were handled in parallel not culture hES cells.
2.2 Culturing of Human Embryonic Stem Cell Line
chHES-3
chHES-3 was one of the several hES cell lines
established in our laboratory[1]. The hES cells were grown on hEFs in a culture medium consisting of 85% Knock-out Dulbecco’s modified Eagle’s medium (KO- DMEM), 15% Knock-out serum replacement, 1 mmol/L L-glutamine, 0.1 mmol/L β-mercaptoethanol, 1% non-essential amino acids, and 4 ng/mL human basic fibroblast growth factor (hbFGF) (all Gibco Invitrogen products, USA). Cultures were passaged once a week by incubation in 200 U/mL collagenase IV (Gibco Invitrogen, USA) for 5-10 min at 37 ℃ and seeded on freshly feeder layer. Re-feed was every other day.
2.3 RT-PCR
Differential Expression of Wnts, β-catenin and E-cadherin in hEFs and Normal,
Abnormal Karyotype hES Cells during Culture invitro
485
M-MuLV revers trancriptase (200 U/µL). The reaction was terminated by heating at 70 ℃ for 10 min and then kept at 4 ℃. The PCR primer pairs of 19 Wnts,
β-catenin and E-cadherin were synthesized as following:
Wnt1-F: 5′-tactacgttgctactggcactga-3′,
Wnt1-R: 5′-cctctgttgccgtaaaggac-3′;
Wnt2-F: 5′-ggctaacgagaggtttaagaagc-3′,
Wnt2-R: 5′-ttgagaaagctcctttgagacac-3′;
Wnt2b-F: 5′-tgtatatgccatctcatcagcag-3′,
Wnt2b-R: 5′-tccacagtatttctgcattcctt-3′;
Wnt3-F: 5′-ccaatctcaagtggactttgttc-3′,
Wnt3-R: 5′-gtgcatgtggtccaggatagt-3′;
Wnt3a-F: 5′-cccactcggatacttcttactcc-3′,
Wnt3a-R: 5′-tcgtacttgtccttgaggaagtc-3′;
Wnt4-F: 5′-cactgaaggagaagtttgatggt-3′,
Wnt4-R: 5′-ttgttactccaccttaggtctgc-3′;
Wnt5a-F: 5′-agtgcaatgtcttccaagttct-3′,
Wnt5a-R: 5′-cagcatgtcttcaggctacat-3′;
Wnt5b-F: 5′-cactctgcctcacaaaggtctat-3′,
Wnt5b-R: 5′-attcagtttagggctttcctgac-3′;
Wnt6-F: 5′-agaagctgcctccatttcg-3′,
Wnt6-R: 5′-gtatccagaggcctttagactgg-3′;
Wnt7a-F: 5′-actctcatgaacttgcacaacaa-3′,
Wnt7a-R: 5′-agttgagggctctgagagatttt-3′;
Wnt7b-F: 5′-agagcaaagtgatgaggagactg-3′,
Wnt7b-R: 5′-acgcccagctaatttttgtattt-3′;
Wnt8a-F 5′-agaactgtagcatgggtgactt-3′,
Wnt8a-R 5′-gcagtaatacttgctcaccaca-3′;
Wnt8b-F 5′-caattcctctgtgctctcctaga-3′,
Wnt8b-R: 5′-agaggaacaaagatcctttggag-3′;
Wnt9a-F: 5′-tcaaggagactgccttcctctat-3′,
Wnt9a-R: 5′-actccacatagcagcaccaac-3′;
Wnt9b-F: 5′-cctcaagtacagcaccaagtttc-3′,
Wnt9b-R: 5′-gcatgcatgtgatgacagtct-3′;
Wnt10a-F: 5′-cgagtcggagctgtgtgtc-3′,
Wnt10a-R: 5′-gtaagcggtgcagcttccta-3′;
Wnt10b-F: 5′-caagagtttcccccactctct-3′,
Wnt10b-R: 5′-cttacacacattcacccactctg-3′;
Wnt11-F: 5′-ctatttgcttgacctggagagag-3′,
Wnt11-R: 5′-cggtctgtgtaggggttgtag-3′;
Wnt16-F: 5′-gcaccaaagagacagcatttatt-3′,
Wnt16-R:5′-tagcagcaccagatgaacttaca-3′;
Beta-catenin-F:5′-atgctgaaacatgcagttgtaa-3′,
Beta-catenin-R:5′-ttgcattccaccagcttctaca-3′; E-cadherin-F:5′-gtgaacacctacaatgccgcca-3′, E-cadherin-R: 5′-ccaaatccgatatgttattttc-3′;
β-actin-F: 5′-ctcctgaagaaggggcgtctaaa-3′,
β-actin-R:5′-ggaagagcttcagggtagggaca-3′.
At last PCR reaction condition is: the RNA isolated from different hEFs and hESCs were reversely transcribed into cDNA. Then the amplification reaction was performed in a 20 µL reaction mixture containing 13.55 µL deionized water, 2 µL 10 × PCR reaction buffer, 2 µL 2.5 mM dNTP, 0.4 µL Taq DNA polymerase, 0.45 µL primer pair mixture (0.5 µg/µL) and 1.6 µL cDNA, in a programmable thermal cycler, PE 9700: initial denaturation at 95 ℃ for 90 s, and then 35 cycles of 94 ℃ for 40 s, 60 ℃ for 40 s and 72 ℃ for 58 s. The extension step in the last cycle was 72 ℃ for 5 min, and the mixture was finally kept at 4 ℃. The PCR products were then separated on a 2.0% agarose gel and analyzed using β-actin as contrast. The products of PCR were sequenced.
3. Results
3.1 Analysis of Normal and Abnormal Karyotype of hES Cells
The analysis of the caryotype of chHES-3 hES cell line showed that the caryotype of chHES-3 hES cells was normal after 27 passages, but the caryotype of
chHES-3 hES cells was abnormal after 34 passages with duplication of 1p32-1p36 area.
3.2 Differential Expression of Wnts, β-catenin and E-cadherin in hEFs after Culture Normal and
Abnormal Karyotype hES Cells
Fig. 1 Wnts, β-catenin and E-cadherin expression in hEFs after culture normal and abnormal karyotype hES cells. A: control hEFs after culture hEFs six days using CM (condition medium); B: hEFs after culture normal hES cells six days
using CM; C: hEFs after culture abnormal hES cells six days using CM.
Fig. 2 Wnts, β-catenin and E-cadherin expression in normal and abnormal hES cells.
only in hEFs after culture abnormal karyotype hES cells six days (Fig. 1C). Wnt7a was detected only in hEFs after culture normal karyotype hES cells six days (Fig. 1B). The expression level of Wnt 9a was detected very high in control hEFs (Fig. 1A), but very low in hEFs after culture normal and abnormal hES cells (Fig. 1B and Fig. 1C).
3.3 Differential Expression of Wnts, β-catenin and E-cadherin in Normal and Abnormal hES Cells
Wnts, β-catenin and E-cadherin gene expressions were assessed in normal hES cells (Fig. 2) and abnormal hES cells (Fig. 2). Results showed that expression of Wnt3,
Wnt9a and Wnt10b were detected weakly in normal hES cells, but higher in abnormal hES cells. At the same, Wnt3a, Wnt4, Wnt5b, Wnt7a, Wnt8a and Wnt11
were only expressed in abnormal hES cells. At the same time, E-cadherin was expressed in normal hES cells and disappeared in abnormal karyotype hES cells.
4. Discussion
So as to filter the canndidate Wnts, β-catenin and
E-cadherin genes that may maintain hES cells normal karyotype or urge hES cells karyotypic change. In this study, RT-PCR results showed Oct 4 and Nanog were expressed in normal hES cells (data not shown), that indicated that normal and abnormal hES cells have hES cells characterization. The analysis of the caryotype of
chHES-3 hES cell line was showed that the caryotype of chHES-3 hES cells was normal after 27 passages, but the caryotype of chHES-3 hES cells was abnormal after 34 passages with duplication of 1p32-1p36 area. The results of analysis of Wnts, β-catenin and
E-cadherin differential expression in hEFs after culture normal and abnormal karyotype hES cells showed
Wnt3 was detected only in hEFs after culture abnormal karyotype hES cells six days (Fig. 1C), which suggests
Differential Expression of Wnts, β-catenin and E-cadherin in hEFs and Normal,
Abnormal Karyotype hES Cells during Culture invitro
487
cells and Wnt7a was detected only in hEFs after culture normal karyotype hES cells six days (Fig. 1B), which suggests Wnt7a was needed for culture normal karyotype hES cells on hEFs. Wnt7a may be important for culture normal hES cells. The expression level of
Wnt 9a was detected very high in control hEFs (Fig. 1A), but very low in hEFs after culture normal and abnormal hES cells. hES cells growth may need low level Wnt9a
expression. Analysis of Wnt differential expression in normal and abnormal hES cells showed that Wnt3,
Wnt9a and Wnt10b were detected weakly expressed in normal hES cells (Fig. 2), but higher in abnormal hES cells (Fig. 2). At the same, Wnt3a, Wnt4, Wnt5b, Wnt7a,
Wnt8b and Wnt11 were expressed in abnormal hES cells (Fig. 2). At the same time, E-cadherin was disappeared in abnormal karyotype hES cells (Fig. 2) compared with normal karyotype hES cells (Fig. 2). In many tissues, activation of Wnt signaling has also been associated with cancer [11]. Recent studies have shown that both Wnt signaling and cadherin-mediated cell-cell adhesion are important in the organization and maintenance of stem cells [12]. Unchecked Wnt signaling [13] and/or the loss of cell-cell adhesion [14-15] are involved in cancer induction and progression. Loss of cadherin expression can also promote tumorigenesis [14-15]. So Wnt3, Wnt9a and
Wnt10b high expressed in hES cells and E-cadherin loss may urge hES cell karyotype change.
In summary, our results demonstrated that Wnt7a
was needed for culture normal karyotype hES cells and
Wnt3 was needed for culture abnormal karyotype hES cells on hEFs; high expression of Wnt3, Wnt9a and
Wnt10b in hES cells and absence of E-cadherin may cause hES cells karyotype change.
Acknowledgment
This work was supported by grants from the National Nature Science Foundation of China (No.31071091; No.30971570) and Department of Education key project of Hunan Province, China (09A035 and 07B029).
References
[1] C.Q. Xie, G. Lin, K.L. Luo, S.W. Luo, G.X. Lu, Newly expressed proteins of mouse embryonic fibroblasts irradiated to be inactive, Biochemical and Biophysical Research Communications 315 (2004) 581-588.
[2] J.A. Thomson, J. Itskovitz-Eldor, S.S. Shapiro, M.A. Waknitz, J.J. Swiergiel, V.S. Marshall, et al., Embryonic stem cell lines derived from human blastocysts, Science 282 (1998) 1145-1147.
[3] B.E. Reubinoff, M.F. Pera, C.Y. Fong, A. Trounson, A. Bongso, Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro, Nat. Biotechnol. 18 (2000) 399-404.
[4] C.H. Xu, M.S. Inokuma, J. Denham, K. Golds, P. Kundu, J.D. Gold, et al., Carpenter, Freeder-free growth of undifferentiated human embryonic stem cells, Nat. Biotechnol.19 (2001) 971-974.
[5] F.P. Martin, Unnature selection of cultured human ES cells, Nature Biotechnology 22 (2004) 42-43.
[6] J.S. Draper, K. Smith, P. Gokhale, H.D. Moore, E. Maltby, J. Johnson, et al., Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells, Nat. Biotechnol. 22 (2004) 53-54.
[7] K. Willert, J.D. Brown, E. Danenberg, A.W. Duncan, I.L. Weissman, T. Reya, et al., Wnt proteins are lipid-modified and can act as stem cell growth factors, Nature 423 (2003) 448-452.
[8] W.J. Nelson1, R. Nusse, Convergence of Wnt, catenin, and cadherin pathways, Science 303 (2004) 1483-1487. [9] K.M. Cadigan, R. Nusse, Wnt signaling: A common
theme in animal development, Genes Dev. 11 (1997) 3286-3305.
[10] C. Jamora, E. Fuchs, Intercellular adhesion, signalling and the cytoskeleton,Nature Cell Biol.4 (2004) 101-108. [11] T. Reya1, H. Clevers, Wnt signalling in stem cells and
cancer, Nature 434 (2005) 843-850.
[12] A. Gonzalez, J. Reyes, Stem cells, niches and cadherins: A view from Drosophila,Cell Sci. 116 (2003) 949-954. [13] P. Polakis, Wnt signaling and cancer, Genes Dev. 14
(2000) 1837-1851.
[14] J.P. Thiery, Epithelial-mesenchymal transitions in tumour progression, Nature Rev. Cance.2 (2002) 442-454. [15] R.A. Pagliarini, T. Xu, A genetic screen in Drosophila
for metastatic behavior, Scienc.302 (2003) 1227-1231. [16] J.B. Lee, J.M. Song, J.E. Lee, J.H, Park, S.J. Kim, S.M.
Identification, Cloning and Characterization of
Dictyoglomus Turgidum
CelA, an Endoglucanase with
Cellulase and Mannanase Activity
Phillip J. Brumm, Spencer Hermanson, Joshua Luedtke and David A. Mead
C5-6 Technologies, 2120 W. Greenview Drive, Middleton, WI 53562, USA
Received: September 28, 2010 / Accepted: March 01, 2011 / Published: July 30, 2011.
Abstract: The discovery of new, highly active, biomass-degrading enzymes is important to the development of a sustainable biofuels industry. Dictyoglomus turgidum,a thermophilic, anaerobic eubacterium that ferments cellulose and produces ethanol and hydrogen, was chosen as a candidate to screen for novel enzymes. A novel thermostable endoglucanase, CelA, was identified and purified during screening of a shotgun library of Dictyoglomus turgidum and subsequently subcloned and expressed in E. coli. The celA gene coding for a 312 amino acid protein showed low homology to proteins outside the genus Dictoglomi and lacked an apparent signal peptide. CelA had a broad substrate range, possessing both endo and exo activity on soluble and insoluble β-(1,4)-linked glucose-containing substrates as well as endo activity on soluble and insoluble β-(1,4)-linked mannose containing substrates. The specific activity of CelA was 226 U/mg using β-glucan, 66 U/mg using glucomannan, and 63 U/mg using CMC as substrates. The high temperature optimum of 70 ℃ to 80 ℃ and wide substrate range of the enzyme might make it an excellent tool for biomass degradation at high temperature.
Key words: Cellulase, mannanase, thermophilic, biomass degradation, Dictyoglomus turgidum.
1. Introduction
Cellulose-containing plant cell walls provide an abundant and renewable source of glucose, pentose, and other small carbon compounds, many of which have significant commercial value. Accordingly, there has been substantial interest in developing improved techniques for enzymatic processing of cellulosic materials. Thermophilic cellulases are of considerable interest because of the potential benefits provided by the use of high temperature enzymes and organisms. Benefits of high temperature enzymes include higher specific activity that decreases the amount of enzyme needed, enhanced enzyme stability allows improved hydrolysis performance over time, high temperature operation reduces or eliminates microbial
Corresponding author: Phillip J. Brumm, Ph.D., Chief Scientific Officer, research fields: carbohydrate chemistry and enzymology. E-mail: [email protected].
contamination problems, and improved flow and loading of solid materials, all leading to improvement of the overall economy of the process. However, growth on cellulosic substrates and production of thermostable cellulases is rare among thermophilic microorganisms. A review of the literature (summarized in Ref. [1]) revealed only a few truly thermophilic organisms reported to produce cellulases.
Dictyoglomus species represent a novel group of thermophilic, anaerobic organisms with considerable biotechnological promise; these organisms are so unique that they have been given their own genus,
Dictoglomi. Dictyoglomus turgidum (originally named
Dictyoglomus turgidus [2]) is one of only two accepted members of the genus Dictoglomi; another being
Dictyoglomus thermophilum [3]. Dictyoglomus
Identification, Cloning and Characterization of Dictyoglomus Turgidum CelA, an Endoglucanase with Cellulase and Mannanase Activity
489
temperatures makes the identification and characterization of the cellulytic enzymes from Dictyoglomus turgidum
worthwhile. This could result in a better understanding of microbial strategies for decomposing biomass and improved catalysts for conversion of carbohydrates to C5 and C6 sugars. In this report we describe unique properties of an unusual carbohydrase CelA.
2. Materials and Methods
2.1 Materials
Dictyoglomus turgidum strain 6724T bacterial cell concentrate was a kind gift of Dr. Frank T. Robb, Center of Marine Biotechnology, University of Maryland Biotechnology Institute. Dictyoglomus turgidum strain 6724T is on deposit at Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH. 10G electro-competent E. coli cells, BL21 (DE3) chemically competent E. coli cells, pEZSeq (a lac promoter vector), Clostridium thermocellum CelA (CthCelA), CelG (CthCelG), CelI (CthCelI), CelO (CthCelO), and CAZyme β-Glucosidase 1 were obtained from Lucigen, Middleton, WI. pET28a vector was obtained from Merck Chemicals, San Diego, CA.
Pure polysaccharides, azurine cross-linked-labeled, and D-Glucose (GOPOD Format) Assay Kit were obtained from Megazyme International (Wicklow, Ireland). 4-methylumbelliferyl-β-D-cellobioside (MUC), 4-methylumbelliferyl-β-D-xylopyranoside (MUX), and 4-methylumbelliferyl-β-D-glucoyranoside (MUG) were obtained from Research Products International Corp. (Mt. Prospect, IL). Octyl Sepharose Fast Flow, Q Sepharose Fast Flow, and Sephacryl S-100 High Resolution column media were purchased from GE Healthcare Life Sciences (Piscataway, NJ). CelLytic IIB reagent, pNP-β-glucoside, pNP-β-cellobioside, 4-methylumbelliferyl-β-D-lactopyranoside (MUL), carboxymethyl cellulose (CMC) and Avicel PH-101 cellulose powder were purchased from Sigma-Aldrich (St. Louis, MO). Acid swollen cellulose was prepared as described in Ref. [5]. All other chemicals were of analytical grade.
2.2 Growth of Organisms
YT plate media was used in all molecular biology screening experiments and Terrific Broth was used for liquid cultures.
2.3 Enzyme Assays
The endo-glucanase specificity of CelA was determined in 0.50 mL of 50 mM acetate buffer, pH5.8, containing 0.2% azurine cross-linked-labeled (AZCL) insoluble substrate and 1.0 µg of enzyme protein. Assays were performed at 70 ℃, with shaking at 1000 rpm, for 20 minutes in a Thermomixer R (Eppendorf, Hamburg, Germany). Tubes were clarified by centrifugation and absorbance values determined using a Bio-Tek ELx800 plate reader. The exo-glucanase
specificity of CelA was determined by spotting 1.0 µg of enzyme directly on agar plates containing 10 mM 4-methyl umbelliferyl substrate. Plates were incubated in a 70 ℃ incubator for 60 minutes; after incubation, the plates were examined using a hand-held UV lamp and compared to negative and positive controls.
Enzyme specific activity was measured using a micro version of the Modified Somogyi Method for reducing sugars as described in Ref. [6]. The reaction mixtures containing 200 µL of substrate (1% β-glucan or other carbohydrate in 50 mM acetate buffer, pH 5.8) and 5 µL enzyme sample were incubated at 70 ℃ for 10 minutes. Micromoles of sugars formed were determined using a glucose standard curve, and unit activity calculated as micromoles of reducing sugar per minute per milligram of protein.
Format) Assay Kit. The temperature optimum of CelA was determined using the reducing sugar assay with 2% β-glucan as substrate at pH 5.8. The pH optimum of CelA was determined using the reducing sugar assay with 1% β-glucan as substrate at 70 ℃.
HPLC analysis of hydrolysis products was carried out on a Shimadzu HPLC equipped with a Rezex ROA organic acid column operating at 60 ℃. Elution was achieved using a mobile phase of 0.005 N H2SO4 at a
flow rate of 0.6 mL/min. Products were detected using a refractive index detector and identified using a series of known cellodextrin standards.
2.4 Library Construction and Screening
A cell concentrate of Dictyoglomus turgidum was lysed using a combination of SDS and proteinase K, and genomic DNA was purified using phenol/chloroform extraction [7]. The genomic DNA was precipitated, treated with RNase to remove residual contaminating RNA, and fragmented by hydrodynamic shearing (HydroShear apparatus, GeneMachines, San Carlos, CA) to generate fragments of 2-4 kb. The fragments were purified on an agarose gel, end-repaired, and ligated into pEZSeq, a lac promoter vector. To identify cellulases, the D. turgidum library was transformed into 10G electro-competent E. coli cells and screened on
YT plates containing 30 μg/mL kanomycin and 100
μg/mL MUC. Positive (blue-fluorescing) cells were picked, re-streaked, and grown overnight at 37 ℃ in
2.0 mL of TB supplemented with 30 μg/mL kanomycin. The cultures were collected by centrifugation and the pellets were lysed by incubation in 200 μL of CelLytic
IIB reagent for 30 minutes at 37 ℃. The lysates were
clarified by centrifugation and 50 μL aliquots were
assayed at 70 ℃ in 0.5 mL of 50 mM acetate buffer,
pH 5.8 containing either 0.2% AZCL-HE-Cellulose for cellulase activity or 0.2% AZCL-Arabinoxylan for hemicellulase activity. The lysate of one MUC-positive culture, designated CelA, also released soluble dye from AZCL-HE-Cellulose, indicating that CelA expressed an endo-cellulase.
2.5 Enzyme Purification
E. coli cells containing the Dtur celA gene were grown overnight at 37 ℃ in 2 L of TB containing 30
μg/mL kanomycin. Cells, 20.3 g, were resuspended in 100 mL of 50 mM Tris-HCl, pH8.0, and lysed by sonication. The lysate was clarified by centrifugation and E. coli proteins were precipitated by heat treatment at 80 ℃ for 15 minutes. The heat-treated lysate was clarified by centrifugation. The clarified material (90 mL) was diluted with an equal volume of 4 M (NH4)2SO4 and was then applied to a 30 mL Octyl
Sepharose Fast Flow column equilibrated with 2 M (NH4)2SO4. The column was washed with 120 mL of
2 M (NH4)2SO4 in 100 mM Tris-HCl, pH7.5, and eluted
sequentially with 200 mL total gradient of a) 2 M (NH4)2SO4 to 0 M (NH4)2SO4 in 100 mM Tris-HCl, pH
7.5 and b) 0% to 60% propylene glycol in 100 mM Tris-HCl, pH7.5. Active fractions were identified by hydrolysis of AZCL-HE-Cellulose, pooled, diluted 1:1 with deionized water, and applied to a 15 mL Q Sepharose Fast Flow column equilibrated with 50 mM Tris-HCl, pH 8.0. The column was washed with 20 mL of 50 mM Tris-HCl, pH8.0, and the enzyme was eluted with a 100 mL gradient of 0 to 1,000 mM NaCl in 50 mM Tris-HCl, pH8.0. Active fractions were pooled and concentrated to 2.0 mL. The concentrate (0.8 mL) was diluted with 0.2 mL of 80% glycerol and applied to a 150 mL Sephacryl S-100 High Resolution column equilibrated with 50 mM Tris-HCl, pH 8.0. Active fractions were pooled and concentrated to 1.0 mL for characterization studies.
SDS denaturing gel electrophoresis was used to verify the purity and size of the cellulase. Electrophoresis was performed on a 4-20% gradient acrylamide gel. The results (Fig. 1) show an approximately 37 kDa band in all fractions.
2.6 Subcloning and Expression
Identification, Cloning and Characterization of Dictyoglomus Turgidum CelA, an Endoglucanase with Cellulase and Mannanase Activity
491
A B B C C D D E E E Fig. 1 SDS PAGE of Dtur CelA purification samples. Legend (from left to right): A: Molecular weight markers; B: E. coli lysate, 1× and 3×; C: Heat-treated lysate, 1× and 3×; D: Concentrated Q sepharose active fractions, 1× and 3×; E: Concentrated S-100 active fractions, 1×, 3× and 5×.
reading frame of the correct size beginning with a
methionine codon. To determine the open reading frame, the N-terminal sequence of the purified protein
was determined by Edman degradation. The sequence obtained was: MNNLPIKRGINFGDALEAPY.
Using 50 nanograms of template plasmid DNA, the putative cellulase gene was amplified using the following expression primers:
CelA Forward:
5’-AACAATCTTATTAAGAGAGGAATTAATTTT-3’;
CelA Reverse:
5’-TCATATATTCCTTTCAGGTATTAATGCCCT-3’.
The amplified sequence corresponded to a 936 bp
open reading frame encoding a 37,002 Dalton protein.
The amplified PCR product was cloned into the
pET28a vector. The ligated product was then
transformed into BL21 (DE3) chemically competent
cells and the transformed clones were selected on
plates containing 30 μg/mL kanamycin and 200 μg/mL
4-methylumbelliferyl-β-D-cellobioside. Eight fluorescent transformants were picked, grown in 50 mL cultures,
and induced with 1 mM IPTG. Lysates of the cultures
were prepared and the expressed enzymes were shown
to have properties identical to the original purified
sample of Dtur CelA.
2.7 Bioinformatics
InterProScan Family analysis (http://www.ebi.ac.uk/ Tools/InterProScan/), and BLASTP (Basic Local Alignment Search Tool [8]) (http://blast.ncbi.nlm. nih.gov/Blast.cgi) analysis tools were used to compare CelA with other proteins in the database. Phylogeny analysis was performed by using software at http://www.phylogeny.fr/version2_cgi/index.cgi. Multiple alignments were run using ClustalW [9] alignment with curation to remove positions with gaps [10]. Construction of the phylogenetic tree was obtained using PhyML [11], and graphically displayed using TreeDyn (http://www. treedyn.org/). Operon predictions [12] were run using http://www.microbesonline.org/operons/OperonList.html. Glycosyl hydrolase predictions were obtained from http://www.cazy.org/geno/acc_geno.html. Signal sequence predictions were determined using (http://www.cbs.dtu.dk/services/SignalP/) [13].
The GenBank accession number of the sequence reported in this paper is GeneID: 7083332. The complete Dictyoglomus turgidum genome is available at NCBI accession number NC_011661 and the complete Dictyoglomus thermophilum genome is available at GenBank accession number CP001146.
3. Results
cellulase Dtur_0276 (68% amino acid identity and 82% amino acid similarity over 312 amino acids).
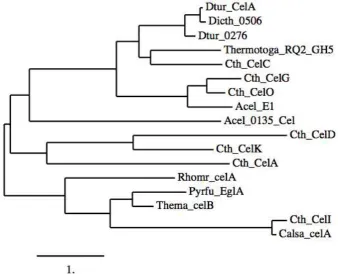
Multiple sequence alignment of the translated Dtur celA gene compared to cellulases from thermophilic organisms shows that CelA is not closely related to other cellulases or endo-glucanases (Fig. 2).
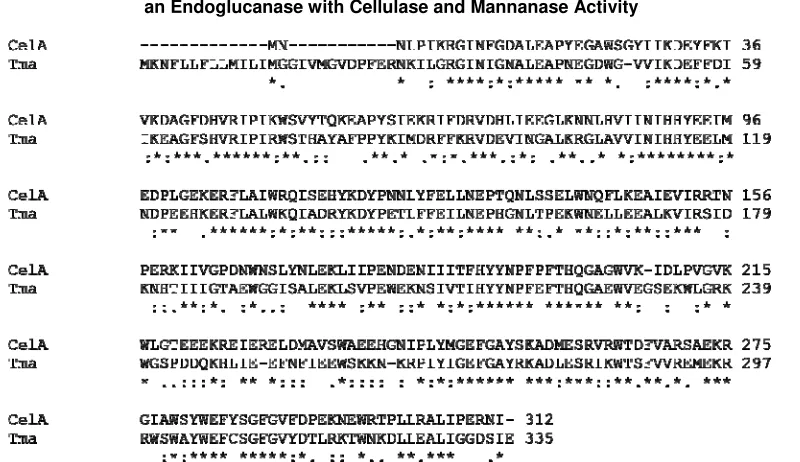
Outside the genus Dictoglomi, Dictyoglomus turgidum CelA is most homologous to an electronically annotated Family 5 glycoside hydrolase of Thermotoga maritima RQ2 (UniProt Accession Number: MSB8 B1LAS2_THESQ); the homology is low, with 52% amino acid identity and 68% amino acid similarity over 312 amino acids (Fig. 3).
Dtur CelA had a temperature optimum between 70 ℃ and 80 ℃; the activity of the enzyme dropped
to approximately 30% of this maximum value at 90 ℃
(Fig. 4). CelA exhibited activity over the pH range of 4.0 to 6.8, with maximum activity between pH 5.6 and 6.8. The activity of the enzyme was not stimulated by addition of either calcium or DTT, and was not inhibited by EDTA.
The endo-glucanase specificity of CelA was determined using insoluble AZCL substrates and normalized to the highest activity (100%). CelA showed highest activity on AZCL-HE-Cellulose (100%), followed by AZCL-β-glucan (55%). The enzyme had low levels of activity on AZCL-arabinoxylan, AZCL-xyloglucan and AZCL-galactaomannan (approximately 4% each). The enzyme had no activity on the following substrates: AZCL-rhamnoglacturanon,
Fig. 2 Phylogenetic comparison of thermostable cellulases analyzed by ClustalW.
Dtur_0276, Dictyoglomus turgidum GeneID: 7083099; Dtur CelA, Dictyoglomus turgidum GeneID: 7083332;
Dicth_0506, Dictyoglomus thermophilum GeneID: 6946331; Cth_CelC, Clostridium thermocellum GeneID: 12584559; Cth_CelG, Clostridium thermocellum GeneID: 462211; Cth_CelO, Clostridium thermocellum GeneID: 7208816; Cth_CelK, Clostridium thermocellum GeneID: 2978565; Cth_CelD, Clostridium thermocellum GeneID: 40671; Cth_CelA, Clostridium thermocellum GeneID: 144752; Cth_CelI, Clostridium thermocellum GeneID: 7208809; Thema_celB, Gene Thermatoga maratima ID: 1297062; Thermotoga_RQ_GH5, Thermatoga RQ2 Gene ID: 6092506; Acel_E1, Acidothermus cellulolyticus Gene ID: 1708075; Acel_0135_Cel, Acidothermus cellulolyticus Gene ID: 4485572; Rhomr_celA, Rhodothermus marinus Gene ID: 2304961; Pyrfu_EglA, Pyrococcus furiosus Gene ID: 5870829;
Identification, Cloning and Characterization of Dictyoglomus Turgidum CelA, an Endoglucanase with Cellulase and Mannanase Activity
493
Fig. 3 ClustalW alignment of Dictyoglomus turgidum CelA with Tma, the electronically annotated Family 5 glycoside hydrolase of Thermotoga sp. RQ2.
Fig. 4 Temperature-activity curve for Dtur CelA.
Activity of Dictyoglomus turgidum cellulase as a function of temperature at pH5.8 using 1% beta-glucan as substrate as described in Materials and Methods.
AZCL-curdlan, AZCL-galactan, or AZCL-arabinan. CelA hydrolyzed MUC, MUL and MUG substrates, indicating that the enzyme possessed at least a minimal exo-activity on glucans. No activity was detectable on MUX.
Dtur CelA showed significant activity on five soluble and three insoluble polymeric substrates (Table 1). The highest specific activity, 226 U/mg was obtained using β-glucan as substrate. Enzymatic activity was observed with both Avicel and ASC using a coupled assay (CelA plus b-glucosidase) to remove any product inhibition by cellobiose. The values for cellulose and
Table 1 Specific activity of Dtur CelA.
Substrate Specific activity (U/mg)
β-glucan 226
Glucomannan 66
CMC 63
Xyloglucan 3.3
Galactomannan 2.0
Mannan 1.8
ASC 0.59 Avicel 0.01 Xylan n.d. pNP-β-cellobioside 16
pNP-β-glucoside n.d.
n.d.: not detectable, less than 0.01 U/mg of activity.
ASC are estimates based on low dosages of enzyme, the enzyme did not show a linear response between dosage and product formation with these two substrates. ASC hydrolysis was carried out for 65 hr at 60 ℃. At 10% conversion of ASC to reducing sugars, the major products formed were glucose (1.5%) and cellobiose (8.5%); no cellotriose or cellotetraose was detectable.
4. Discussion
percentage of CAZymes present in the genome, 2.35% of its total genes, higher than the percentage present in the cellulose-degrading, thermophilc anaerobe,
Clostridium thermocellum, with 2.2% of its total genes (Garret Suen, University of Wisconsin, Madison, private communication). This apparent strong degradation capacity, coupled with the uniqueness of the organism, suggested that Dictyoglomus turgidum
would be an excellent source of new and novel biomass-degrading enzymes.
Dictyoglomus turgidum CelA was isolated from plate screening of a shotgun library in E. coli. The enzyme was purified, its gene sequenced and subcloned into pET28a for high level expression. Subsequent sequencing of the Dictyoglomus turgidum
genome indicated the CelA protein corresponded to the predicted Dtur_0670 gene product. Amplification and cloning of the Dtur_0670 gene product directly from genomic DNA and cloning into pET28a gave a protein with molecular weight and properties identical to CelA, confirming the identification (data not shown).
Structurally, Dictyoglomus turgidum CelA is a novel cellulase, showing little structural similarity to known cellulases or other proteins outside the genus
Dictoglomi. Noticeably absent from the enzyme is a signal peptide sequence, suggesting either an intracellular location for the enzyme or an alternate route for secretion of the protein. Unlike some cellulases active on cellulose, CelA does not contain any dockerin domains, and there is no evidence from the genome of Dictyoglomus turgidum for the existence of other cellulosomal components such as scaffoldins. Therefore, it is unlikely that the
Dictyoglomus turgidum CelA is a component of a larger cellulytic structure. In contrast to many noncomplexed cellulases, Dictyoglomus turgidum
CelA does not contain an identifiable carbohydrate binding domain separate from the hydrolytic domain of the enzyme. This is further supported by the small size of the enzyme, 312 A.A., significantly smaller than C. thermocellum CBM-containing cellulases such as CelI
(887 A.A.) or CelO (660 A.A.). The observation that
Dictyoglomus turgidum CelA is able to hydrolyze insoluble cellulose substrates without the presence of either an identifiable carbohydrate binding domain (CBD) or cellulosomal structure suggests that the enzyme possesses an alternative mechanism for the recognition and binding to these substrates. Alternatively, the enzyme could be an intracellular protein that breaks down oligosaccharides imported into the cell.
Functionally, Dictyoglomus turgidum CelA is a novel cellulase, active on a much wider range of substrates than other cellulases. The enzyme has broad substrate specificity, being able to hydrolyze both native and dye-linked substrates containing either
β-(1,4)-linked glucose and β-(1,4)-linked mannose residues. The specific activity of the enzyme for
β-(1,4)-linked glucose-containing substrates is similar to that reported for cloned Clostridium thermocellum
cellulases (Table 2).
The enzyme does not cleave AZCL-curdlan, indicating it does not recognize β-(1,3)-linked glucose residues. The high activity on β-glucan, CMC, and glucomannan (which contains a backbone of
β-(1,4)-linked glucose and mannose residues in a 5:8 ratio) indicates cleavage between β-(1,4)-linked glucose residues appears to be preferred. Steric hindrance by the high degree of xylose substitution on the glucose backbone of xyloglucan may explain the relatively low rate of hydrolysis of this substrate. Hydrolysis of mannan and galactomannan indicates the ability of CelA to recognize and cleave both soluble
Table 2 Comparison of Dtur CelA activities to various Cth
cellulytic enzymes.
CMC β-glucan ASC Avicel Source
Dtur CelA 63 226 0.59 0.01 This work
Cth CelA 9.7 48 3.5 0.08 Schwarz [14]
Cth CelI 12.2 n.d. 0.94 0.17 Gilad [15]
Cth CelQ 159 392 1.4 0.40 Arai [16]
Cth CelJ 74 49 0.092 0.055 Arai [17]
Identification, Cloning and Characterization of Dictyoglomus Turgidum CelA, an Endoglucanase with Cellulase and Mannanase Activity
495
(galactomannan) and insoluble (mannan) substrates between β-(1,4)-linked mannose residues. This wide range of substrate utilization is not common among thermophilic cellulases; pure Clostridum thermocellum
cellulases CthCelA (Glycosyl Hydrolase, Family 8), CthCelC (Glycosyl Hydrolase, Family 5), CthCelG (Glycosyl Hydrolase, Family 5), CthCelI (Glycosyl Hydrolase, Family 9), CthCelK (Glycosyl Hydrolase, Family 9), CthCelL (Glycosyl Hydrolase, Family 5), and CthCelO (Glycosyl Hydrolase, Family 5) showed no activity on either AZCL-galacomannan or AZCL-xyloglucan, even at high enzyme dosing (data not shown).
The enzyme possesses significant exoglucanase activity along with its endoglucanase activities. The high specific activity on pNP-β-cellobioside, coupled with the product distribution observed with ASC, suggests that Dictyoglomus turgidum CelA can be classified as a processive endo-cellulase.
Only a handful of genes have been expressed and characterized from Dictyoglomi, including: xylanase
xynB [4] beta-mannanase manA [19], xylanase xynA [20], and amylases amyA, B and C [21, 22]. In D. thermophilum, almost all amylase activity is found in the media, although AmyA and AmyB lack a typical signal sequence at their amino terminal end [22]. ManA [4] does not have an apparent signal peptide. Xylanase XynA [20] and xylanase xynB [4] from D. thermophilum possess signal peptides that appear to be functional in E. coli.
Dictyoglomus thermophilum, the type species of this genus, was described as growing only on soluble substrates [3], while Dictyoglomus turgidus, obtained originally from Uzon Caldera, was found to grow weakly on solid polysaccharides, including microcrystalline cellulose [2]. A preliminary analysis from the recent whole genome sequencing of these two species reveals that both have a similarly large number of glycosyl hydrolases (Dtu, 54; Dth, 55 [www.cazy.org]), few of which have a recognizable signal peptide (data not shown). Both strains also
possess a novel growth condition where large spherical aggregates containing over 100 cells and surrounded by a common outer membrane can be seen [2, 3].This observation and the apparent lack of signal sequences on many secreted proteins suggest the presence of some unknown mechanism of protein secretion in this gram-negative thermophile.
The high temperature optimum and wide substrate range of Dictyoglomus turgidum CelA may make it an excellent tool for biomass degradation at high temperature as well as an excellent model for studies on cellulase structure and function. The discovery of this novel cellulase suggests that Dictyoglomus turgidum may be an exciting source of new and novel enzymes for biomass conversion and other applications. The recent completion of the genome sequence of
Dictyoglomus turgidum, with 54 potential CAZyme genes, should provide a foundation for the functional annotation of the many other enzymes this bacterium utilizes to degrade plant polysaccharides.
5. Conclusions
Screening a genomic library of Dictyoglomus turgidum resulted in the discovery of a novel thermostable endoglucanase enzyme, CelA. The enzyme shows low homology to other cellulases and endoglucanases previously described. The enzyme utilize a broader range of substrates than other thermophilic cellulases and endoglucanases, suggesting the enzyme evolved to degrade a broad range of polymeric substrates found in low concentrations in the hot spring environment. This broad range of substrate utilization, combined with the high temperature optimum of 70 ℃ to 80 ℃, makes this enzyme a useful tool for degrading a range of biomass polymers at elevated temperature. The unusual structure of the molecules makes it an excellent candidate for further structural characterizations and comparisons.
Acknowledgments
DE-FG36-06GO16106, “Novel enzyme products for the conversion of defatted soybean meal to ethanol” and funded in part by the DOE Great Lakes Bioenergy Research Center (DOE Office of Science BER DE-FC02-07ER64494).
References
[1] L.R. Lynd, P.J. Weimer, W.H. van Zyl, I.S. Pretorius, Microbial cellulose utilization: Fundamentals and biotechnology, Microbiology and Molecular Biology Reviews 66 (2002) 506-577.
[2] V.A. Svetlichnii, T.P. Svetlichnaya, Dictyoglomus turgidus sp. nov., a new extremely thermophilic eubacterium isolated from hot springs of the Uzon volcano caldera, Mikrobiologiya 57 (1988) 435-441.
[3] T. Saiki, Y. Kobayashi, K. Kawagoe, T. Beppu,
Dictyoglomus thermophilum gen. nov., sp. nov., a chemoorganotrophic, anaerobic, thermophilic bacterium, Int. J. Syst. Bacteriol. 35 (1985) 253-259.
[4] D.D. Morris, M.D. Gibbs, C.W. Chin, M.H. Koh, K.K. Wong, R.W. Allison, et al., Cloning of the xynB gene from
Dictyoglomus thermophilum Rt46B.1 and action of the gene product on kraft pulp, Appl. Environ. Microbiol. 64 (1988) 1759-1765.
[5] S. Zhou, L.O. Ingram, Simultaneous saccharification and fermentation of amorphous cellulose to ethanol by recombinant Klebsiella oxytoca SZ21 without supplemental cellulose, Biotechnology Letters 23 (2001) 1455-1462.
[6] D. Mead, J. Boyum, C. Drinkwater, K. Gowda, D. Stevenson, P. Weimer, et al., Functional annotation of fibrobacter succinogenes S85 carbohydrate active enzymes, Appl. Biochem. Biotechnol. 163 (5) (2010) 649-657. [7] J. Sambrook, E.F. Fritsch, T. Maniatis, Molecular Cloning:
A Laboratory Manual, Cold Spring Harbor Laboratory Press, NY, 1989.
[8] S.F. Altschul, T.L. Madden, A.A. Schäffer, J. Zhang, Z. Zhang, W. Miller, et al., Gapped BLAST and PSI-BLAST: A new generation of protein database search programs, Nucleic Acids Res. 25 (1997) 3389-3402.
[9] J.D. Thompson, D.G. Higgins, T.J. Gibson, CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice, Nucleic Acids Res. 22 (1994) 4673-4680.
[10] S. Guindon, O. Gascuel, A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood, Syst. Biol. 52 (2003) 696-704.
[11] A. Dereeper, V. Guignon, G. Blanc, S. Audic, S. Buffet, F. Chevenet, et al., Phylogeny.fr: robust phylogenetic
analysis for the non-specialist, Nucleic Acids Res. 36 (2) (2008) 465-469.
[12] M.N. Price, K.H. Huang, E.J. Alm, A.P. Arkin, A novel method for accurate operon predictions in all sequenced prokaryotes, Nucleic Acids Research 33 (2005) 880-892. [13] O. Emanuelsson, S. Brunak, G. von Heijne, H. Nielsen,
Locating proteins in the cell using TargetP, SignalP, and related tools, Nature Protocols. 2 (2007) 953-971.
[14] W.H. Schwarz, F. Grabnitz, W.L. Staudenbauer, Properties of a Clostridium thermocellum endoglucanase produced in Escherichia coli, Applied and Environmental Microbiology 51 (1986) 1293-1299.
[15] R. Gilad, L. Rabinovich, S. Yaron, E.A. Bayer, R. Lamed, H.J. Gilbert, et al., CelI, a noncellulosomal family 9 enzyme from Clostridium thermocellum, is a processive endoglucanase that degrades crystalline cellulose, J. Bacteriol. 185 (2003) 391-398.
[16] T. Arai, H. Ohara, S. Karita, T. Kimura, K. Sakka, K. Ohmiya, Sequence of CelQ and properties of CelQ, a component of the Clostridium thermocellum cellulosome, Appl. Microbiol. Biotechnol. 57 (2001) 660-666.
[17] T. Arai, R. Araki, A. Tanaka, S. Karita, T. Kimura, K. Sakka, et al., Characterization of a cellulase containing a Family 30 carbohydrate-binding module (CBM) derived from Clostridium thermocellum CelJ: Importance of the CBM to cellulose hydrolysis, J. Bact. 185 (2003) 504-512. [18] V.V. Zverlov, G.A. Velikodvorskaya, W.H. Schwarz, A newly described cellulosomal cellobiohydrolase, CelO, from Clostridium thermocellum: Investigation of the exo-mode of hydrolysis, and binding capacity to crystalline cellulose, Microbiol.148 (2002) 247-255. [19] M.D. Gibbs, R.A. Reeves, A. Sunna, P.L. Bergquist,
Sequencing and expression of a beta-mannanase gene from the extreme thermophile Dictyoglomus thermophilum Rt46B.1, and characteristics of the recombinant enzyme, Curr. Microbiol. 39 (1999) 351-357. [20] M.D. Gibbs, R.A. Reeves, P.L. Bergquist, Cloning,
sequencing, and expression of a xylanase gene from the extreme thermophile Dictyoglomus thermophilum
Rt46B.1 and activity of the enzyme on fiber-bound substrate, Appl. Environ. Microbiol. 61 (1995) 4403-4408. [21] S. Fukusumi, A. Kamizono, S. Horinouchi, T. Beppu,
Cloning and nucleotide sequence of a heat-stable amylase gene from an anaerobic thermophile, Dictyoglomus thermophilum, Eur. J. Biochem. 174 (1988) 15-21. [22] S. Horinouchi, S. Fukusumi, T. Ohshima, T. Beppu,
Journal of Life Sciences 5 (2011) 497-502
Evaluation of Barley Genotypes for Resistance to
Pyrenophora Teres
Using Molecular Markers
Leona Leisova-Svobodova1, Lenka Stemberková2, Martina Hanusová2 and Ladislav Kučera1
1. Department of Molecular Biology, Crop Research Institute, Drnovska 507, Prague 16106, Czech Republic 2. Department of Barley Breeding, Selgen, Jankovcova 1, Prague 17037, Czech Republic
Received: December 17, 2010 / Accepted: February 17, 2011 / Published: July 30, 2011.
Abstract: Net blotch disease caused by the fungus Pyrenophora teres belongs to the complex of leaf blotch diseases that decrease the yield and the quality of barley (Hordeum vulgare L.). This study deals with the use of microsatellites localized nearby the quantitative trait loci, associated with the resistance to P. teres to screen barley lines and varieties that could be introduced into a net blotch resistance breeding program. Thirty-five barley microsatellite loci and 65 barley genotypes, 265 alleles were detected. The number of alleles per locus ranged from 2 to 26. The arrangement of genotypes into clusters based on microsatellite data does not correspond to their resistance level to P. teres even though chosen microsatellite markers have been localized nearby QTLs associated with the resistance to P. teres.
Key words: Barley, microsatellites,net blotch, resistance.
1. Introduction
Net blotch is one of the barley (Hordeum vulgare L.) leaf spot diseases threatening the yield and quality every year. It is caused by Pyrenophora teres, anamorph Drechlera teres (Sacc.) Shoem, occurring in two forms: P. teres f. teres - net form and P. teres f.
maculata - spot form [1].
The resistance to net blotch is polygenic [2, 3] and several QTL (Quantitative Trait Loci) mapping studies focused on the resistance to net blotch were done on several mapping populations. Microsatellite markers EBmac0874, Bmag0173, HVM074, Bmag0807, Bmag0496 (chromosome 6H), EBmac0906, Bmag0306, Bmac0310 Bmac0181 (chromosome 4H), Bmac0067 (chromosome 3H), Bmag0381 (chromosome 2H) and HVM0043 (chromosome 1H) showed a good co-segregation with net-type, net blotch resistance [2-7].
Resistance to spot form net blotch, which is caused
Corresponding author: Leona Leisova-Svobodova, Ph.D., research field: molecular biology. E-mail: [email protected].
by P. teres f. sp. maculata, has not been studied as extensively as resistance to net form net blotch. A major QTL was found on the long arm of the 4H chromosome and a co-segregating SSR marker HVM0067 explained 64% of phenotypic variation [6].
The main aim of this study was to use microsatellite markers showing a significant co-segregation with resistance genes to the net blotch disease to screen barley lines and varieties that could be introduced into the net blotch resistance breeding program.
2. Material and Methods
PCR products were analyzed using the method of capillary electrophoresis on a sequencer ABI PRISM 3130 (Applied Biosystems, USA). LIZ500 (Applied Biosystems, USA) was used as a size standard. Electrophoretograms were processed by GeneMapper software (Applied Biosystems, USA) and binary data matrices were built. A dissimilarity matrix was computed in DARwin software using the Jaccard coefficient [12, 13]. A dendrogram was constructed using an unweighted neighbour joining method. Bootstrap analysis with 1000 replicates was performed to estimate the robustness of the tree. Population structure was also studied using the software
STRUCTURE (version 2.2) to support the DARwin data [14]. An exact binomial test for goodness-of-fit was performed to determine whether the presence of allele correlated with the field evaluation for a barley genotype (the null hypothesis). Statistical significances were tested by the exact binomial test of goodness-of-fit using an EXCEL spreadsheet calculator (http://udel.edu/~mcdonald/statexactbin.html).
3. Results and Discussion
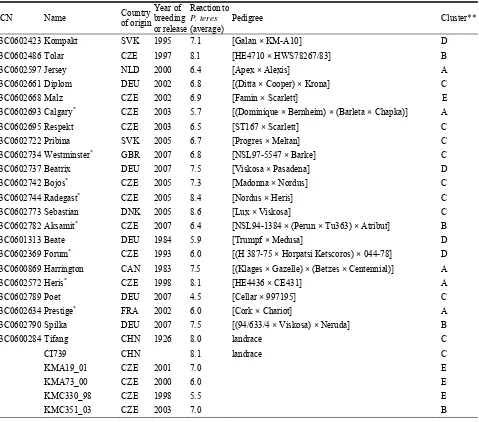
A set of barley varieties and lines (65 accessions) (Table 1) were evaluated using 35 microsatellite markers. Twelve microsatellites were selected because
Table 1 Barley varieties and lines used in the study.
ECN Name Country
of origin Year of breeding or release
Reaction to
P. teres
(average)
Pedigree Cluster**
03C0602423 Kompakt SVK 1995 7.1 [Galan × KM-A10] D
03C0602486 Tolar CZE 1997 8.1 [HE4710 × HWS78267/83] B
03C0602597 Jersey NLD 2000 6.4 [Apex × Alexis] A
03C0602661 Diplom DEU 2002 6.8 [(Ditta × Cooper) × Krona] C
03C0602668 Malz CZE 2002 6.9 [Famin × Scarlett] E
03C0602693 Calgary* CZE 2003 5.7 [(Dominique × Bernheim) × (Barleta × Chapka)] A
03C0602695 Respekt CZE 2003 6.5 [ST167 × Scarlett] C
03C0602722 Pribina SVK 2005 6.7 [Progres × Meltan] C
03C0602734 Westminster* GBR 2007 6.8 [NSL97-5547 × Barke] C
03C0602737 Beatrix DEU 2007 7.5 [Viskosa × Pasadena] D
03C0602742 Bojos* CZE 2005 7.3 [Madonna × Nordus] C
03C0602744 Radegast* CZE 2005 8.4 [Nordus × Heris] C
03C0602773 Sebastian DNK 2005 8.6 [Lux × Viskosa] C
03C0602782 Aksamit* CZE 2007 6.4 [NSL94-1384 × (Perun × Tu363) × Atribut] B
03C0601313 Beate DEU 1984 5.9 [Trumpf × Medusa] D
03C0602369 Forum* CZE 1993 6.0 [(H 387-75 × Horpatsi Ketscoros) × 044-78] D 03C0600869 Harrington CAN 1983 7.5 [(Klages × Gazelle) × (Betzes × Centennial)] A
03C0602572 Heris* CZE 1998 8.1 [HE4436 × CE431] A
03C0602789 Poet DEU 2007 4.5 [Cellar × 997195] C
03C0602634 Prestige* FRA 2002 6.0 [Cork × Chariot] A
03C0602790 Spilka DEU 2007 7.5 [(94/633/4 × Viskosa) × Neruda] B
03C0600284 Tifang CHN 1926 8.0 landrace C
CI739 CHN 8.1 landrace C
KMA19_01 CZE 2001 7.0 E
KMA73_00 CZE 2000 6.0 E
KMC330_98 CZE 1998 5.5 E
Evaluation of Barley Genotypes for Resistance to Pyrenophora Teres Using Molecular Markers
499
(Table 1 Continued).
KMC420_00 CZE 2000 6.5 E
KMD294_01* CZE 2001 8.0 A
KMD413_03 CZE 2003 7.5 B
KMD473_03 CZE 2003 6.0 B
KMD535_98 CZE 1998 7.0 E
KME713_98 CZE 1998 6.5 E
KMF888_03 CZE 2003 5.5 B
KMI1586_03 CZE 2003 7.0 B
KMJ1260_00* CZE 2001 5.0 C
KMJ1317_01* CZE 2001 6.0 C
KMJ(F2)00-570-02*CZE 2002 6.0 D
KMJ(F2)00-575-02*CZE 2002 7.0 D
KMK(F2)00-596-02 CZE 2002 6.5 B
KML(F2)00-610-02 CZE 2002 5.0 B
KML839_95 CZE 1995 4.0 E
KMO949_95 CZE 1995 7.0 E
KMP1934_01* CZE 2001 5.5 C
ST10535_04* CZE 2004 4.0 [Akcent × [Derkado × F1 (Profit × (D × 1B-86B)]] E
ST10539_04* CZE 2004 5.0 [Akcent × (Derkado × F1 (Profit × (D × 1B-86B))] E ST10837_04 CZE 2004 7.0 [Amulet × (Portia × (RS20-1 × Kieb B))] D
ST11536_04 CZE 2004 6.5 [Kompakt × KML1711/98] E
ST1182_04 CZE 2004 5.5 [Aspen × KMJ1450/98] E
ST12526_04 CZE 2004 8.0 [Annabell × (Dallas × (Jubilant × (Mla19 × Kriničnyj)))] C ST1332_03 CZE 2003 7.0 [HE7872 × (Chariot × (Mla20RS145-39 × KiebB))] B
ST1458_03 CZE 2003 3.0 [KME682/97 × Ria] C
ST14675_04 CZE 2004 7.0 [Amulet × KMI827/96] A
ST1515_05 CZE 2005 6.0 [Amulet × KMI827/96] A
ST1517_05 CZE 2005 7.0 [Amulet × KMI827/96] E
ST1640_03* CZE 2003 4.0 [Akcent × (Perun × (Mla17 × Kriničnyj))] E
ST16743_04 CZE 2004 7.0 [KMA73/00 × SG-S 268] B
ST16839_04 CZE 2004 7.5 [KMB192/00 × Annabell] C
ST1705_04 CZE 2004 5.0 [Annabell × KML1711/98] C
ST17234_04* CZE 2004 4.0 [NSL98-1867 × KMJ1260/00] D
ST1960_04 CZE 2004 5.5 [KME1587/92 × HE6621] A
ST1964_04 CZE 2004 4.5 [Ceb9538 × (RS1-8-151 × Picollo)] A
ST820_03* CZE 2003 6.5 [Famin × KMA87/96] D
ST823_03 CZE 2003 6.5 [Famin × KMC120/96] E
ST825_03* CZE 2003 5.0 [Famin × KMI827/96] E
*
Genotypes possessing mlo-11 allele; ** Cluster of the tree (Fig. 1) the genotype is located.
they showed a significant co-segregation with QTL associated with the resistance to P. teres. They are predominantly located on the chromosomes 4H and 6H (EBmac0906, Bmac0181, Bmac0310, Bmag0306, HVM74, Bmag0496, Bmag0807, Bmag0173,
ranged from 2 to 26, with an average of 7.6 alleles per locus in the range of analyzed genotypes.
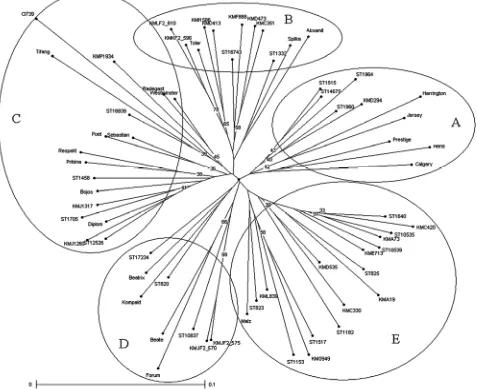
Cluster analysis based on microsatellite data showed that genetic diversity in the studied set of barley varieties and lines is rather high and that the grouping of genotypes into clusters has low reliability (Fig. 1). Similarly, the results of the model based on cluster analysis (STRUCTURE) support a high heterogeneity of the evaluated set and only a small part of genotypes (18, i.e. 28%) was possible to assign into inferred clusters with the 90% level of probability.
Five main clusters with low bootstrap support were identified (Fig. 2). All of them involved varieties and lines originated from different countries, released in
different years, with a different field evaluation for the resistance to P. teres (Table 1, Figs. 1 and 2). Cluster A is formed mainly of modern varieties including foreign genotypes and comprise three susceptible barleys ‘Jersey’, ‘Prestige’ and ‘Calgary’ together with resistant barley ‘Heris’. Cluster B consists of breeding lines originated mainly from Kromeriz, further from two cultivars ‘Tolar’ and ‘Spilka’ with a higher level of resistance and barley ‘Aksamit’ belonging to genotypes with a lower level of resistance. For this cluster, the presence of markers BMS18-139, Bmag0496-192 and HVM74-186 localised on the chromosome 6H is characteristic. However, the presence of these markers was not detected only in genotypes
Evaluation of Barley Genotypes for Resistance to Pyrenophora Teres Using Molecular Markers
Fig. 2 Results of the evaluation of spring barley varieties resistance to P. teres in field experiments (locality Stupice, year 2008 and 2009). Verticals represent 0.95 interval of reliability, mean least square.
with a higher level of resistance to P. teres. Resources of resistance to P. teres are involved in cluster C together with modern cultivars with higher resistance (from 8.6 to 7.3) ‘Sebastian’, ‘Radegast’, ‘Bojos’, ‘Westminster’, ‘Poet’, ‘Respekt’, ‘Pribina’, and ‘Diplom’. A lot of barley accessions within this cluster have Annabel and Krona in their pedigree. Cluster D contains modern barleys ‘Beatrix’, ‘Kompakt’, ‘Forum’ and an older one ‘Beate’. Two of them were more susceptible in field experiments - ‘Forum’ and ‘Beate’. Cluster E consists mainly of Czech breeding lines having ‘Famin’, ‘Akcent’ and ‘Salome’ in their pedigree. The Manchurian landrace ‘CI739’ generally considered as a source of the resistance to the net blotch disease was found in cluster C together with another landrace from Manchuria ‘Tifang’ and several modern varieties.
The results obtained within the scope of this work indicated that the net blotch - barley pathosystem is more complex than previously assumed. Although
several major resistance or susceptibility genes control the resistance of barley, complex interactions between the host and pathogen also play an important role [7]. Barley varieties as well as P. teres populations have proven to have a high level of genetic diversity throughout the world [15-17]. Therefore QTLs are effective only against some isolates and it may be assumed that they control partial resistance in an isolate-specific manner [18]. Grewal et al. [19] screened Canadian and Australian barley lines for their resistance to Canadian P. teres isolates. They found that the majority of Australian barley mapping population parents was susceptible to Canadian P. teres
isolates, i.e. suggesting markers linked to their resistance may not be useful for Canadian breeding.