Journal of Life Sciences
Volume 8, Number 12, December 2014 (Serial Number 80)
Dav i d
Publication Information
Journal of Life Sciences is published monthly in hard copy (ISSN 1934-7391) and online (ISSN 1934-7405) by David Publishing Company located at 240 Nagle Avenue #15C, New York, NY 10034, USA.
Aims and Scope
Journal of Life Sciences, a monthly professional academic journal, covers all sorts of researches on molecular biology, microbiology, botany, zoology, genetics, bioengineering, ecology, cytology, biochemistry, and biophysics, as well as other issues related to life sciences.
Editorial Board Members
Dr. Stefan Hershberger (USA), Dr. Suiyun Chen (China), Prof. Dr. Fadel Djamel (Algeria), Dr. Francisco Torrens (Spain), Dr. Filipa João (Portugal), Dr. Masahiro Yoshida (Japan), Dr. Reyhan Erdogan (Turkey), Dr. Grzegorz Żurek (Poland), Dr. Ali Izadpanah (Canada), Dr. Barbara Wiewióra (Poland), Dr. Amanda de Moraes Narcizo (Brasil), Dr. Marinus Frederik Willem te Pas (The Netherlands), Dr. Anthony Luke Byrne (Australia), Dr. Xingjun Li (China), Dr. Stefania Staibano (Italy), Prof. Dr. Ismail Salih Kakey (Iraq), Hamed Khalilvandi-Behroozyar (Iran).
Manuscripts and correspondence are invited for publication. You can submit your papers via Web Submission, or E-mail to [email protected] or [email protected]. Submission guidelines and Web Submission system are available online at http://www.davidpublishing.com.
Editorial Office
240 Nagle Avenue #15C, New York, NY 10034, USA
Tel: 1-323-9847526, 1-302-5977046; Fax: 1-323-9847374, 1-323-9080457 E-mail: [email protected], [email protected]
Copyright©2014 by David Publishing Company and individual contributors. All rights reserved. David Publishing Company holds the exclusive copyright of all the contents of this journal. In accordance with the international convention, no part of this journal may be reproduced or transmitted by any media or publishing organs (including various websites) without the written permission of the copyright holder. Otherwise, any conduct would be considered as the violation of the copyright. The contents of this journal are available for any citation. However, all the citations should be clearly indicated with the title of this journal, serial number and the name of the author.
Abstracted / Indexed in
Database of EBSCO, Massachusetts, USA Chemical Abstracts Service (CAS), USA
Database of Cambridge Science Abstracts (CSA), USA Database of Hein Online, New York, USA
Ulrich’s Periodicals Directory, USA Universe Digital Library S/B, Proquest
Chinese Database of CEPS, American Federal Computer Library center (OCLC), USA China National Knowledge Infrastructure, CNKI, China
Chinese Scientific Journals Database, VIP Corporation, Chongqing, China Index Copernicus, Index Copernicus International S.A., Poland
Google Scholar (scholar.google.com)
Subscription Information
Price (per year): Print $420, Online $300, Print and Online $560.
David Publishing Company
240 Nagle Avenue #15C, New York, NY 10034, USA
Tel: 1-323-9847526, 1-302-5977046; Fax: 1-323-9847374, 1-323-9080457 E-mail: [email protected]
David Publishing Company www.davidpublishing.com
DAV ID P UBL ISH IN G
J LS
Journal of Life Sciences
Volume 8, Number 12, December 2014 (Serial Number 80)
Contents
Microbiology
925 Antibacterial Activity of Lyngbya and Chroococcus Species Isolated from Koya (Hizoop River)
Sewgil Saaduldeen Anwer and Parween Mohsin Abdulkareem
931 Low Pressure Cold Plasma as an Alternative Method for Black Pepper Sterilization
Maciej Grabowski, Agnieszka Strzelczak and Waldemar Dąbrowski
940 Exopolysaccharides from Lactic acid Bacteria as Corrosion Inhibitors
Ignatova-Ivanova Tsveteslava and Radoslav Ivanov
Botany and Zoology
946 Morphogenesis of Oil Palm (Elaeis guineensis Jacq.) Fruit in Seed Development
Hermine Bille Ngalle, Joseph Martin Bell, Georges Franck Ngando-Ebongue, Hernild Eman-Evina,
Godswill Ntsefong Ntsomboh and Armand Nsimi-Mva
955 Characterization in Greenhouse Conditions of Two Salt Tolerant Citrumelo (Citrus paradisi Macf.
x Poncirus trifoliata (L.) Raf.) Cultivars
Anas Fadli, Ouiam Chetto, Abdelhak Talha, Rachid Benkirane, Raphaël Morillon and Hamid Benyahia
Interdisciplinary Researches
967 Economic Viability of Production of Tree Paricá (Schizolobium amazonicum Huber ex. Ducke) of
Reforestation Project in the Municipality Paragominas-PA, Brazil
Manoel Tavares de Paula, Altem Nascimento Pontes, Hélio Raymundo Ferreira Filho, Lucy Anne Cardoso Lobão Gutierrez, Ismael Matos da Silva, Maria da Conceição Silva Damasceno and Aline Lima de Sena
972 Electric Energy Saving
Journal of Life Sciences 8 (2014) 925-930 doi: 10.17265/1934-7391/2014.12.001
Antibacterial Activity of
Lyngbya
and
Chroococcus
Species Isolated from Koya (Hizoop River)
Sewgil Saaduldeen Anwer and Parween Mohsin Abdulkareem
Department of Biology, Faculty of Health and Science, University of Koya, Erbil 44001, Iraq
Received: December 15, 2014 / Accepted: December 28, 2014 / Published: December 30, 2014.
Abstract: In the study cyanobacterial strains were isolated from different sites of Hizoop rivers, Koya-Iraq and identified according to their morphological characters by using microscope, two genera which were in filamentous form identified as Chroococcus sp. and
Lyngbya sp.. After identification of genera their optimum growth condition studied by using the effect of temperature and pH to their dry weight. In the result, the optimum temperature and pH for both filamentous cyanobacteria were 25 °C and pH 7.5. Both cyanobacterial strains were extracted with ethanol, methanol and diethyl ether at various concentrations (0.2 g/mL, 0.1 g/mL, 0.005 g/mL) which exhibited the antibacterial activities against Staphylococcus aureus, E. coli and B. subtilus. Inhibition activities of the two cyanobacterial extracts were more effective at high concentration against the tested pathogens at the low concentration, especially those of Lyngbya sp. The higher inhibition zone showed with extract by ethanol.
Key words: Cyanobacteria, pH, temperature, antibacterial activity.
1. Introduction
Cyanobacteria are the most prokaryotic algae and they are found in virtually every type of environment including terrestrial, fresh water, marine habitats. Since cyanobacteria are prokaryotes, they lack membrane bound organelles however the external structure can change from unicellular or colonial to branched or unbranched and filamentous [1]. Like rhodophytes, the cyanophytes possess no flagellated or ciliated cell at any stage of their life cycle. They are heavily pigmented with chlorophyll a, beta carotene, and several xanthophylls [2, 3].
The bacterial infections are still the major problem in the world today, because these bacteria which causes disease will eventually develop ways to resist the drugs as well. To help preventing and treating these illnesses, many researchers have studied the antimicrobial effects of various plants extracts, as well as antimicrobial activity of algae.
Corresponding author: Sewgil Saaduldeen Anwer, associate professor, research field: industrial microbiology. E-mail: [email protected].
In general, isolation of bioactive compounds from cyanobacteria is done to discover new compounds. A number of cyanobacteria produce toxins that may have potential pharmaceutical application [4]. The authors had found that various strains of cyanobacteria are known to produce intracellular and extracellular metabolites with diverse biological activities such as antibacterial, antialgal, antifungal and antiviral activity [5-8]. As an efficient strategy of investigation, organic solvents have been used to extract the possible lipid soluble active principles from microalgae [8, 9]. The antimicrobial substances involved may target various kinds of microorganisms, prokaryotes as well as eukaryotes. The properties of secondary metabolites in nature are not completely understood [6, 10].
2. Materials and Methods
2.1 Isolation and Identification
Samples were collected from sites along the Hizoop rivers Fig. 1. Water samples diluted and plated onto plates of BG11 medium solidified with %1.5 agar-agar. According to Rippka and Castenholz et al.,
D
the cultures were incubated under continues light at pH 7.2 and 23 °C. Two weeks later, following the growth of colonies on the agar media, the colonies were removed with pasture micropipettes and were gently blown into liquid medium then incubated at 23 °C, at pH 7.2 [11, 12].
After 15 days single cells and filamentous removed with pasture micropipette and examine under light microscope and identified as described by [10, 13, 14].
2.2 Effect of Environmental Factors
Strains were cultivated at different temperature 20 °C, 23 °C, 25 °C & 30 °C and different pH 6, 6.5, 7, 7.5, 8 & 8.5 [15, 16].
2.3 Analytical Method
2.3.1 Cell Dry-Weight and Fresh-Weight Estimation According to Oswald, samples grown at 100 mL BG11 medium After 2 weeks 10 mL of samples were taken and cells harvested after centrifugation for 10 min. To determine the cell dry weight, cells were harvested after centrifugation for 10 min, the collected sample were dried in oven at 80 °C and weighed quickly after drying [17].
2.3.2 Preparation of Cyanobacterial Extracts
Cyanobacterial cells were dried at 70 °C, and then the cells were grinded in sterile tubes. As described by Thummajitsakul, cells were mixed with ethanol, diethyl ether and methanol for 1 mL with shaking for
10 min and kept in room temperature for 10 h. After that, the solvent was removed by incubation at 60 °C and redissolved in water (ratio 0.2 g/mL, 0.1 g/mL and 0.05 g/mL) and kept at 4 °C until use for further assay [18].
2.3.3 Antibacterial Bioassay
Staphylococcus aureas, E. coli and Bacillus subtilus, were used as test microorganisms. Antibacterial activity was determined by the disc method as described by ghasemi et al., Filter paper disc were saturated with 20 μL of test bacteria and dried under laminar air flow and placed on nutrient agar, the plates were incubated at 37 °C for 24-28 h. Ampicilin, gentamicin and amphoterin were used as positive control. The diameter of inhibition zones were determined and used as an indication of antibacterial activity [19].
3. Results
Growing cells were observed and photographed with light microscope, and the mode of division of strain examined in slide culture showed the following characters:
Filament erect or less curved, rarely solitary, ends not constricted and not attenuated, cross walls marked with one or two large granules on either side; and is identified as Lyngbya sp. Spherical or ovate colony of 2-4 spherical cells. Evenly arranged cell sheath usually is well defined with colorless lamellate Chroococcus
sp. (Fig. 2).
(a)—Lyngbya sp. (b)—Chroococcus sp.
Fig. 2 Isolated unicellular cyanobacteria.
3.1 Effect of pH to Growth Rate
The higher cell dry weight was at pH 7.5 for two cyanobacteria and the lowest growth rate shown at pH 8.5 for both cyanobacteria isolates (Fig. 3).
3.2 Effect of Temperature to Growth Rate
In Fig. 4, the temperature role in the growth of cyanobacterial strain, at 20 °C and 35°C, the higher growth shown at 25°C for both cyanobacterial strains, while in 20°C, the cyanobacteria strains showed low growth.
3.3 Antibacterial Activity of Chroococcus sp. and
Lyngbya sp.
The effect of ethanol, diethyl ether and methanol extractions of cyanobacteria Lyngbya sp. and
Chroococcus sp. on the inhibition of tested pathogens was shown in Tables 1-3. The results showed that ethanol, diethy ether and methanol extracts of
Lyngbya sp. and Chroococcus sp. at various concentrations (0.2 g/mL, 0.1 g/mL and 0.005 g/mL) exhibited the antibacterial activities against
Staphylococcus aureus, E. coli and B. subtilus.
Inhibition activities of the two cyanobacterial extracts were more effective at high concentration against the tested pathogens at the low concentration, especially
those of Lyngbya sp. The higher inhibition zone showed with extract by Ethanol.
4. Discusion
Cyanobacteria grow well within the temperature range of 25-30 °C, these temperatures displays a short exponential phase, along linear phase and stationary phase from about 14 days on. In this study the optimum temperature for the growth of filamentous and unicellular cyanobacteria were around 20-35°C, the highest growth found at 25 °C, and the lowest growth found at 35 °C which show denaturized of pigment after 10 days of cultivation. This result shows that increase of temperature from 20 °C to 30 °C caused increase of chlorophyll and cell dry weight but higher this value caused decreasing of chlorophyll-a and cell density with cell dry weight. This result is agreed with finding of Donmezet al. [21-23].
The effect of pH on growth rate and chlorophyll content was shown in Fig. Cyanobacteria grow at all pH values just the best growth determined at pH 7.5 for both cyanobacterial strain. This pH is near the pH of isolated place.
Fig. 3 Effect of pH to dry weight of cyanobacterial strains.
Fig. 4 Effect of temperature to dry weight of cyanobacterial strains.
Table 1 Antibacterial activity of Lyngbya sp. and Chroococcus sp.extracted with ethanol (diameter of inhibition zone in mm).
Bacterial species Lyngbya sp.(g/mL) Chroococcus sp.(g/mL)
0.05 0.1 0.2 0.05 0.1 0.2
Escherichia coli 4 7 10 5 7 9
Staphylococcus aureus, 4 9 11 6 9 9
B. subtilus 5 6 9 5 8 10
0 0.5 1 1.5 2 2.5 3 3.5 4 4.5
PH6 g/L PH7 g/L PH7.5 g/L PH8 g/L PH8.5 g/L
Chroococcus sp.
Lyngbya sp.
0 0.5 1 1.5 2 2.5 3 3.5 4
20 ºC 23 ºC 25 ºC 30 ºC 35 ºC
Lyngbya sp.
Chroococcus sp.
Chroococcus sp.
Lyngbya sp. Chroococcus sp.
Table 2 Antibacterial activity of Lyngbya sp. and Chroococcus sp.extracted with diethyl ether (diameter of inhibition zone in mm).
Bacterial species Lyngbya sp.(g/mL) Chroococcus sp.(g/mL)
0.05 0.1 0.2 0.05 0.1 0.2
Escherichia coli 7 9 8 3 6 7
Staphylococcus aureus 9 9 9 5 6 7
B. subtilus 7 9 8 4 7 8
Table 3 Antibacterial activity of Lyngbya sp. and Chroococcus sp.extracted with methanol (diameter of inhibition zone in mm).
Bacterial species lyngbya sp.(g/mL) Chroococcus sp. (g/mL)
0.05 0.1 0.2 0.05 0.1 0.2
Escherichia coli 5 7 9 5 7 8
Staphylococcus aureus, 5 9 8 4 6 7
B. subtilus 6 9 8 6 7 8
Escherichia coli, Staphylococcus aureus and Bacillus subtilus. The extracts of Lyngbya sp. shows the highest inhibition zone to Staphylococcus aureus (11 mM) at 0.2 g/mL which showed more sensitivity to the cyanobacteria extracts than those of Escherichia coli and Bacillus subtilus. The cyanobacterial extracts showed more effective inhibition activities by increasing their concentration. It was reported by Crosby, that correlation between the extracts concentration and the inhibition zone sizes in the logarithm revealed the linear relationship [20].
5. Conclusions and Recommendation
After isolation of cyanobacteria from Hizoop rivers and determination of optimum pH and temperature. It was concluded that the extracts of Lyngbya and
Chroococcus species indicated the potential of antibacterial activity against Escherichia coli,
Staphylococcus aureus and Bacillus subtilus.
Therefore, the basic knowledge may be useful in various applications such as pharmaceutics and agricultures, and for further investigations.
The authors suggested that further work should be performed on the isolation and characterization of the active components responsible for the antibacterial activities need to be evaluated.
Reference
[1] Vashishta, B. R., Sinha, A. K., and Sinh, V. P. 2002.
Botany for Degree Students.
[2] Bold, H. and Wynne, M. 1968. Introduction to the Algae, Structure and Reproduction. Englewood: Clifts.
[3] Adhikary, S. P. 2006. Blue Green Algae. Survival Strategies in Diverse Environment. Jaipur: Pointer Publishers.
[4] Katircioglu, H., Beyatli, Y., Aslim, B., Yukskdaag, Z., and Atici, T. 2006. “Screening for Antimicrobial Agent Production in Freshwater.” Internet. J. Microbiol. 2 (2): 64-71.
[5] Volk, R. B., and Furkert, F. H. 2006. “Antialgal, Antibacterial and Antifungal Activity of Two Metabolites Produced and Excreted by Cyanobacteria during Growth.”
Research in Microbiology 161: 180-186.
[6] Metting, B., and Pyne, J. W. 1986. “Biologically Active Compounds from Microalgae.” Enz. Microbiol. Tech. 8: 386-394.
[7] Patterson, G., Baldwin, C., and Bolis, C. 1993. “Antiviral Activity of Cultured Blue Green Algae (Cyanophyta).” J. Phycol. 29: 125-130.
[8] Satsry, V. M. V. S., and Rao, G. R. K. 1994. “Antibacterial Substances from Marine Algae: Successive Extraction Using Benzene, Chloroform and Methanol.”
Bot. Marina. 37: 357-360.
[9] Schlege, I., Doan, N., Chazal, N., and Smith, G. 1999. “Antibiotic Activity of New Cyanobacterial Isolates from Australia and Asia against Green Algae and Cyanobacteria.” J. Appl. Phycol. 10: 471-479.
[10] Dakshini, K. M. M. 1994. “Algal Allelopathy.” Botanical Rev. 60: 182-196.
[11] Rippka, R. 1988. “Isolation and Purification of Cyanobacteria.” Method of Enzymology 167: 3-27. [12] Castenholz, R., Boom, W., and Gerry, G. 2001. “The
[13] Prescott, G. W. 1963. Algae of the Western Great Lakes Area Michigan: Wm. C. Brown.
[14] Desikachary, T. V. 1968. Cyanophyta, Chrococcales.
New York and London academic press.
[15] Holt, J. G., Rieg, N. R., Smeath, P. H. A., Staley, J. T., and Williams, S. T. 1994. Bergeys Manual of Determinative Bacteriology, 9th ed.
[16] Ratkowsky, D. A., Olley, J., Mcmeekin, T. A., and Ball, B. 1982. “Relationship between Temperature and Growth Rate of Bacterial Culture.” Journal of Bacteriology 149 (1): 1-5.
[17] Becker, E. W. 1994. Micro Algae Biotechnology and Microbiology, Measurement of Algal Growth. Cambridge University press.
[18] Oswald, W. J. 1988. Large-Scale Algal Culture Systems (Engineering Aspect). In Micro Algal Biotechnology ed. Browizka, M. A., and Browizka, L. J. Cambridge University. [19] Thummajitsakul, S., Silprasit, K., and Sittipraneed, S.
2012. “Antibacterial Activity of Crude Extracts of Cyanobacteriaphormidium and Microcoleus Species.”
African Journal of Microbiology Research 6 (10): 2576-2579.
[20] Ghasemi, Y., Yazdi, T. M., Shokravi, S., Soltani, N., and Zarrini, G. 2003. “Antifungal and Antibacterial Activity of Paddy Fields Cyanobacteria from the North of Iran.” J. Islamic Republic of Iran 14: 203-209.
[21] Donmez, G., Obali, A., Ozturk, A., Elmaci, A., and Cakmakci, L. 1999. “Isolation and Abundance of Unicellular Cyanobacteria from Mosquito Development Sites.” Tr. J. of Biology 23: 451-456.
[22] Dhargalkar, U. K. 2004. “Effect of Different Temperature Regimes on the Chlorophyll-A Concentration in Four Species of Antarctic Macro Algae.” Seaweed Res. Utiln.
26: 237-243.
[23] Ilknur, Ak., Cirik, S., and Tolga, G. 2008. “Effect of Light Intensity, Salinity and Temperature on Growth in Camalti Strain of Dunaliella Viridis Teodorsco from Turkey.” J. of Biological Sciences 8: 1356-1359.
Journal of Life Sciences 8 (2014) 931-939 doi: 10.17265/1934-7391/2014.12.002
Low Pressure Cold Plasma as an Alternative Method for
Black Pepper Sterilization
Maciej Grabowski1, Agnieszka Strzelczak2 and Waldemar Dąbrowski1
1. Department of Microbiology and Applied Biotechnology, West Pomeranian University of Technology in Szczecin, Szczecin 70-310,
Poland
2. Department of Food Process Engineering, West Pomeranian University of Technology in Szczecin, Szczecin 70-310, Poland
Received: November 30, 2014 / Accepted: December 10, 2014 / Published: December 30, 2014.
Abstract: It was found out that spices straight from the package are not sterile. The only way to receive sterile spices is to use radiation technology which means to irradiate spices with ionizing radiation. However, this method is quite expensive and raises great resistance of public. And this is the reason why we are interested in implementing plasma technology. The first step of the research was to choose the most appropriate spice. The range of available spices is nearly unlimited, however, we took into account the following ones: sweet paprika, basil, rosemary, saffron, marjoram, thyme and black pepper. Finally, we chose black pepper because it is most often used by butchers to make meat products. It is also called the “King of Spices” or the “Black Gold”. Black pepper is one of the most often used spices in the United States and in Europe. It is important to have sterile black pepper when we aim at ripening products for example ripening sausages or some kinds of cheeses. What is more, it was found out that black pepper has antimicrobial properties, antioxidant effects and also antipyretic and analgesic properties. The aim of the research was to receive sterile spices using low pressure cold plasma with oxygen, nitrogen, air, argon and hydrogen peroxide.
Key words: Black pepper, cold plasma, sterilization.
1. Introduction
In the United States in 2010 the import of spices was about 608 million kilograms including black pepper with the amount of about 73 million kilograms. In Poland the import of pepper prevails among other spices and constitutes about 70%, as well as it accounts for about 80% of the Polish spice market [1, 2].
In 2010 alone the spices imported into the United States and probably in other countries, were infected with the bacteria of the genus Salmonella in up to 6.6% of cases. It should be noted that the survival of these bacteria at 25 °C and relative humidity ≤ 40 is equal to 400 days or more. In addition, in the spices many other types of bacteria may occur [1].
The above mentioned incidents concern only single
Corresponding author: Maciej Grabowski, M.S., research fields: plasma and food technology. E-mail: [email protected].
bacteria in given spices. A major problem occurs when we are dealing with foods containing a lot of unknown species of microorganisms. These types of infections can be caused even by streptococci (family Streptococceae), bacteria of the Listeria genus,
Legionella, Clostridium (especially dangerous are the toxins produced by them), Salmonella (cause salmonellosis), spirochetes of the Leptospira genus, sticks of Brucella, bacteria of the Yersinia genus,
Campylobacter and Helicobacter, a closely related family of Actinomycetaceae, Enterobacteriaceae (including a type of Klebsiella, Proteus, Serratia),
Escherichia coli bacteria, Pseudomonas aeruginosa, as well as non-tuberculosis mycobacteria [3], 4].
Plasma is termed as the fourth state of matter. It displays different properties as compared with a gas, liquid and solid phase, and is formed at the temperatures at which the ionization potential value is exceeded by the mean values of the particles kinetic
D
energy. The change of the physical properties of the gas, such as the loss of the insulation and the appearance of the electrical conductivity properties of the ionized gas, is considered to be the border between the state of the gas and plasma. It should be noted that some authors do not consider the plasma the fourth state of matter, but the “state of dispersion of matter” or a matter in the “potential original state”. This is because the molecules are formed out of the plasma, which in turn enables to define a solid, liquid and gaseous state [5-10].
Plasma is neutrally ionized gas which is composed of interacting particles. These include: atoms, free radicals, electrons, photons, positive and negative ions as well as excited and unexcited molecules. In order to generate plasma, that is ionize the gas, the selected gas, for example oxygen, air, nitrogen or argon, should be subjected to the impact of an electromagnetic field (or a magnetic or electrical one) of high frequency using a direct current (or alternating current) and waves (for example microwaves or radiowaves) [5, 7-9, 11-13].
This process may take place under atmospheric, vacuum or elevated pressure [7-9, 11, 13-16]. Plasma operates in the temperature range which can vary from room temperature to the temperature within the range of energy greater than a few electron volts (1 eV = 1,132,685 oC) [8, 11, 15, 17-19]. Moreover, plasma can be generated either with the thermodynamic equilibrium behavior or without it [8, 11]. In addition to plasma classification according to pressure, temperature and thermodynamic equilibrium, it also divided according to the type of discharge. Each type of the discharge in which the plasma is formed is characterized by different parameters such as frequency, power, the distance of the electrode from the sample, voltage, current, pressure or the type of the gas used [8, 20, 21].
To this day, only one example of the application of cold plasma for microbial decontamination has been found in the available literature, yet it concerns red
pepper powder. No literature has been found on the application of cold plasma for black pepper sterilization, therefore, we may be regarded as the pioneers in this field, and the research is largely justified.
2. Materials and Methods
2.1 Black Pepper Powder
First, black pepper powder (Piper nigrum L.) was obtained from the local store. The next step was to choose a black pepper, from one out of many foreign companies, including American ones, available on the Polish market. The choice was based on the following criteria: color, smell, and friability. The aim was to carry out research on available commercial spices produced by significant companies, in order to work out an alternative method to be used in the future by spice companies for the spice decontamination by means of cold plasma.
2.2 Microbiological Analyses
2.3 Inoculation and Sample Preparation
In the case of using low pressure non-equilibrium cold plasma, all samples were dried in advance. This was due to the fact that the process would take too long without partial drying.
2.4 Low Pressure Cold Plasma System
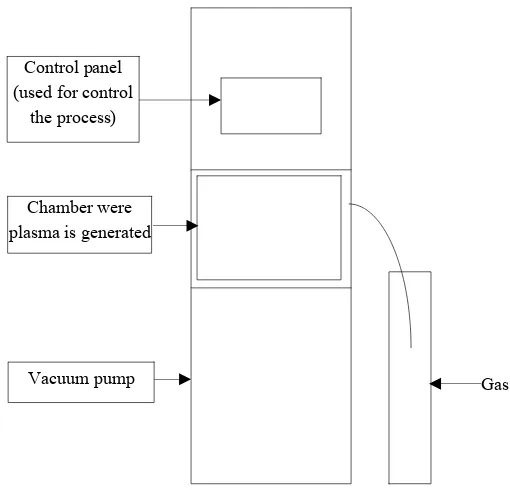
Diener plasma generator (Diener Electronic company) generates low pressure (0.3 mbar) non-equilibrium cold plasma (Fig. 1). The plasma was produced at the radio frequency of 13.56 MHz and the maximum power of 300 Watts. Plasma can be generated either in a continuous or pulsed way. Additionally, a stationary (sample set on a shelf) or a rotary (sample placed in a special jar) variant may be used. The gases selected for the research included oxygen, nitrogen, air and argon. The sterilization time was equal to 15, 30, 45 and 60 min respectively.
What is more, advanced Sterilization Products (Johnson & Johnson Company) plasma generator was also used. In this case, the pressure of up to 9 mbar was applied during the 60 min plasma sterilization by means of hydro peroxide. The plasma was produced at the radio frequency of 13.56 MHz and the maximum
power of 400 Watts. The structure of this generator was identical to the one of Diener device.
At the beginning the sample is put into the chamber. After that, the pressure is lowered to the most desired level. This is the reason why the spices are dried in advance. If the water activity is too high, the vacuum pump, which provides relatively low pressure, needs more time to lower it and thus prolongs the whole process. Then the gas is introduced into the chamber. The gas ionization then starts after which plasma sterilization takes place.
2.6 Temperature, Mass and Water Activity Measurements of Black Pepper Powder
The temperature of the process was measured by means of a built-in temperature sensor and an additional thermometer. The mass was checked with technical weight whereas a special meter was used in order to measure water activity.
2.7 Statistical Analysis
The statistical significance of the low-pressure cold plasma influence on the bactericidal activity of black ground pepper was revealed by means of the U
Fig. 1 Low pressure, cold, non-equilibrium plasma reactor.
Gas Control panel
(used for control the process)
Chamber were plasma is generated
Mann-Whitney nonparametric test due to non-normality of the data set (P < 0.05 in chi-square and Kolmogorow-Smirnow tests). Calculations were performed in Statistica 10 software.
3. Results and Discussion
3.1 Decontamination of the Microorganisms
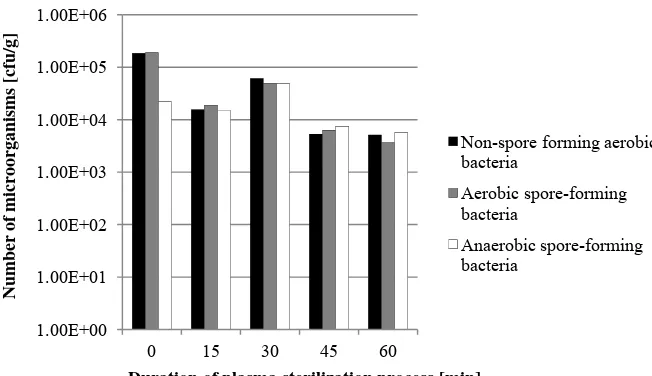
To start with, it needs to be mentioned that the article is based on preliminary results. The numbers of microorganisms in black pepper powder after plasma sterilization with oxygen, nitrogen, air and argon are given in Figs. 2-5.
In the case of hydrogen peroxide application, after 60 min the decrease of non-spore forming aerobic bacteria was noted from 105 to 102, of aerobic spore-forming bacteria from 105 to 103, and of anaerobic spore-forming bacteria from 104 to 103. After six months’ storage, the number of each type of microorganisms was below a detection limit. It should be mentioned that in the case of the samples undergoing plasma sterilization by use of oxygen, nitrogen, air and argon, the layer thickness was 4.3 mM, whereas 18.5 mM in the case of the sample used for plasma sterilization by use of hydrogen peroxide.
Fig. 2 The number of microorganisms after plasma sterilization by use of oxygen.
Fig. 3 The number of microorganisms after plasma sterilization by use of nitrogen.
1.00E+00 1.00E+01 1.00E+02 1.00E+03 1.00E+04 1.00E+05 1.00E+06
0 15 30 45 60
Number of microorganisms [cfu/g]
Duration of plasma sterilization process [min]
Non-spore forming aerobic bacteria
Aerobic spore-forming bacteria
Anaerobic spore-forming bacteria
1.00E+00 1.00E+01 1.00E+02 1.00E+03 1.00E+04 1.00E+05 1.00E+06
0 15 30 45 60
Number of microorganisms [cfu/g]
Duration of plasma sterilization process [min]
Non-spore forming aerobic bacteria
Aerobic spore-forming bacteria
Fig. 4 The number of microorganisms after plasma sterilization by use of air.
Fig. 5 The number of microorganisms after plasma sterilization by use of argon.
After 60 min, black pepper sterilization process with the use of cold rotating continuous plasma by use of oxygen, the decrease non-spore forming aerobic bacteria was from 105 to 104, of aerobic spore-forming bacteria from 105 to 104, and of anaerobic spore-forming bacteria was below detection limit.
Some researchers infected legumes and grains with
Penicillum spp. and Aspergillus spp.. After this, they tried to decontaminate the samples with low pressure cold plasma. The pressure was between 0.13 mbar and 0.65 mbar, and the used gas was SF6 (sulfur
hexafluoride). After 15 min, the researches obtained
the decrease in the number of microorganisms of three logarithms. What is more, this process did not affect the possibility of growing grains such as wheat [22]. Other researches tried to sterilize Aspergillus parasiticus from nuts’ surfaces by means of SF6 with
five logarithm effectiveness and one logarythm loss after 5 min plasma sterilization with air. The pressure was between 0.13 mbar and 0.65 mbar [17]. The authors claim that SF6 is non toxic gas, however, it
can decompose, especially when discharges come to action, for example SF4 (sulfur tetrafluoride highly toxic), SO2F2 (sulfuryl fluoride—toxic) or S2F10
1.00E+00 1.00E+01 1.00E+02 1.00E+03 1.00E+04 1.00E+05 1.00E+06
0 15 30 45 60
Number of microorganisms [cfu/g]
Duration of plasma sterilization process [min]
Non-spore forming aerobic bacteria
Aerobic spore-forming bacteria
Anaerobic spore-forming bacteria
1.00E+00 1.00E+01 1.00E+02 1.00E+03 1.00E+04 1.00E+05 1.00E+06
0 15 30 45 60
Number of microorganisms [cfu/g]
Duration of plasma sterilization process [min]
Non-spore forming aerobic bacteria
Aerobic spore-forming bacteria
(sulfur decafluoride—highly toxic) [17, 23]. The case of paprika powder is especially interesting. A research group sterilizing the spice under the pressure varying from 5 to 300 mbar, inoculated Aspergillus flavus
spores to red pepper and then lowered it by 2.5 logarithms after 20 min sterilization by means of nitrogen [24].
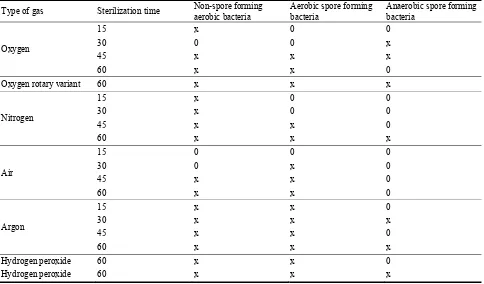
As mentioned above, in the comparative studies, statistical results were obtained by means of a nonparametric test, which is U Mann-Whitney test. Under a detection limit, significant differences observed in each case were the findings. The time of sterilization was different for each applied gas and was equal to 15, 30, 45 and 60 min respectively. Statistically significant differences (P < 0.05) were attested under the following conditions shown in Table 1.
Importantly, both statistically and microbiologically significant findings include all those under the detection limit [25] as well as the ones concerning
non-spore forming aerobic bacteria obtained after 60 min plasma sterilization with oxygen in a stationary variant.
3.2 Mass and Water Activity
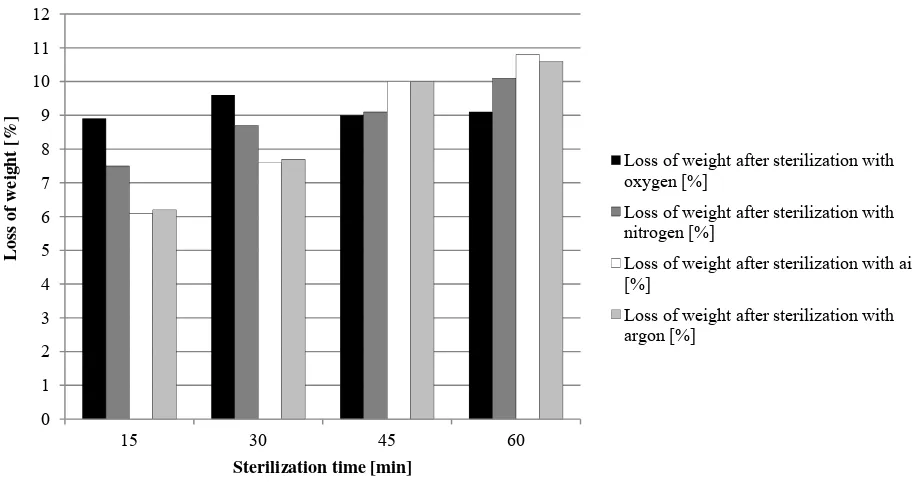
In the case of using low pressure non-equilibrium cold plasma, all samples were dried in advance. This was due to the fact that the process would take too long without drying. Thus, the initial water activity of black pepper averaged 0.510 whereas after three hours’ drying in the laboratory drier at 50 °C and the mill set at 50% of the maximum power, water activity was 0.210 on average. The loss of weight after drying was approximately 0.33 g for the samples with mass of 10 g. Weight losses for ground black pepper are shown in Table 2 and Fig. 6.
After 60 min black pepper sterilization with the use of cold rotating continuous plasma and with the application of air, the loss of the mass of the black pepper after the trial was 2.28 g on average. The water
Table 1 Statistical significant differences in the number of microorganism occurring in black pepper powder after low pressure, cold plasma sterilization process.
Type of gas Sterilization time Non-spore forming aerobic bacteria
Aerobic spore forming bacteria
Anaerobic spore forming bacteria
Oxygen
15 x 0 0 30 0 0 x 45 x x x 60 x x 0
Oxygen rotary variant 60 x x x
Nitrogen
15 x 0 0 30 x 0 0 45 x x 0 60 x x x
Air
15 0 0 0 30 0 x 0 45 x x 0 60 x x 0
Argon
15 x x 0 30 x x x 45 x x 0 60 x x x
Hydrogen peroxide 60 x x 0
Hydrogen peroxide 60 x x x
Fig. 6 Dependence of weight loss of black pepper on sterilization time with low pressure, non-equilibrium, cold plasma.
Table 2 The weight loss of black pepper after the sterilization process with low pressure, non-equilibrium, cold plasma, depending on the duration of the process.
Sterilization time [min] 15 30 45 60
Loss of weight after sterilization with oxygen [g] 0.89 0.96 0.90 0.91 Loss of weight after sterilization with nitrogen [g] 0.75 0.87 0.91 1.01
Loss of weight after sterilization with air [g] 0.61 0.76 1.00 1.08
Loss of weight after sterilization with argon [g] 0.62 0.77 1.00 1.06
Table 3 The temperature of black pepper after the sterilization by means of low-pressure, non-equilibrium cold plasma versus time process.
Sterilization time [min] 15 30 45 60
The temperature after sterilization in an oxygen environment [°C] 28-29 27-28 23-27 19-23 The temperature after sterilization in an nitrogen environment [°C] 20-21 21-23 23-27 27-30 The temperature after sterilization in an air environment [°C] 20-21 21-23 23-26 27-30 The temperature after sterilization in an argon environment [°C] 20 21-22 23-26 26-29
activity after the plasma sterilization process was from about 0.055 for the duration equal to 15 min, up to 0.020 for 60 min. During the sterilization of black pepper with hydrogen peroxide, the mass decrease and the water activity could not have been measured due to the action of the electrostatic forces and the fact that the plasma sterilization packaging constitutes a barrier to bacteria exclusively.
When Aspergillus flavus spores fungi were removed from the red pepper powder, the level of water activity was also lowered. At the deepest point it was even
equal to 0.3, which resulted in the loss of mass [24].
3.3 Temperature Measurement
Because of the fact that low-pressure plasma is also non-equilibrium plasma, the distribution of temperatures was variable (Table 3). During the sterilization by means of low pressure cold plasma in a rotary variant, a similar relationship was observed.
In the case of plazma sterilization with hydrogen peroxide, the temperature of the samples after 60 min was about 25 °C.
0 1 2 3 4 5 6 7 8 9 10 11 12
15 30 45 60
Loss of
w
eight
[%]
Sterilization time [min]
Loss of weight after sterilization with oxygen [%]
Loss of weight after sterilization with nitrogen [%]
Loss of weight after sterilization with air [%]
The temperature generated during the plasma sterilization process in order to eliminate Penicillum
spp. and Aspergillus spp. from grains and legumes was within room temperature to enable their further growth [22]. The temperature in the low pressure chamber generating cold plasma which was used for the sterilization of nuts’ surfaces from Aspergillus parasiticus varied from 20 °C to 30 °C [17]. In the case when low pressure cold plasma was applied on sweet pepper, the temperature was from 23.8 °C to 28.5 °C [24].
3.4 Organoleptic Analysis
Unfortunately, no sensoric panel was used during the research, however, it was noticed that after the application of nitrogen, air and argon, a number of lumps appeared, whereas after the use of oxygen and hydrogen peroxide no lumps occured. In each case neither the change of color nor the loss of sharpness was reported, however, almost the complete loss of smell was observed.
4. Conclusions
The aim of the research was achieved and sterile black pepper was obtained, however, it did not reveal the same organoleptic characteristics as the spice not treated with plasma sterilization. In general, it can be concluded that plasma sterilization offers new opportunities for microbial decontamination of food products as it reduces the number of microorganisms without the application of heat. Sterilization of spices by means of lower pressure cold plasma needs further research since it is does not seem to be the most appropriate method. This method excludes the majority of food products because of the application of low pressure caused by the loss of water. This may result not only in the loss of mass, but also in the food drying out (for example cheese) as well as a very long plasma process.
What is more, very interesting research on the same spice is being carried out but with the application of
atmospheric pressure cold plasma is expected to give much more promising results than low pressure cold plasma.
Acknowledgments
Partly supported by the European Union within the Human Capital Operational Programme, Priority VIII, Activity 8.2, Subactivity 8.2.2; Investment in Knowledge as Regional Development Tool, Contract no. WUP/142/2014 and by the Polish Ministry of Science and Higher Education, Innovation Incubator Project, Contract no. DS/1558/11/W15/POIG/II/2014 (internal no. 5/II/2014).
To anyone interested in the use of plasma for spice sterilization, please meet me at “Natural Products Expo East 2015” Baltimore Convention Center, Baltimore, MD USA on 16-19 September, 2015.
References
[1] FDA 2013. Draft Risk Profile: Pathogens and Filth in Spices 1-213. Report of the Center for Food Safety and Applied Nutrition, Food and Drug Administration, Department of Health and Human Services.
[2] Newerli-Guz, J., and Śmiechowska, M. 2009. “Ocena Zawartości Piperyny w Czarnym Pieprzu Ziarnistym Piperum Nigrum L.” Bromatologia i Chemia Toksykologiczna 3: 827-830.
[3] Bielec, D., and Modrzewska, R. 2007. “Zatrucie Jadem Kiełbasianym Dawniej i Dziś—Aspekty Kliniczne.”
Przegląd Epidemiologiczny 61: 505-512.
[4] Cianciara, J., and Juszczyk, J., eds. 2012. Choroby Zakaźne i Pasożytnicze Tom II. Lublin: Czelej.
[5] Banu, M., Sasikala, P., Dhanapal, A., Kavitha, V., Yazhini, G., and Rajamani, L. 2012. “Cold Plasma as a Novel Food Processing Technology.” International Journal of Emerging trends in Engineering and Development 4: 803-818.
[6] Bryjak, M., Janecki, T., Ganzarz, I., and Smolińska, K. 2009. “Plazmowa Modyfikacja Membran Polimerowych.”
Membrany teoria i praktyka 3: 64-79.
[7] Mrozowski, T. 2007. “Sterylizacja.” Świat farmacji 16: 42-45.
[8] Stryczewska, H. 2009. Technologie plazmowe w energetyce i inżynierii środowiska. Lublin: Wyd. Politechniki Lubelskiej.
217: 41-61.
[10] Szałatkiewicz, J. 2010. “Zastosowanie Plazmy w Technice—Aktualne Tendencje.” Pomiary Automatyka Robotyka 2: 17-20.
[11] Moreau, M., Orange, N., and Feuilloley, M. 2008. “Non-thermal Plasma Technologies: New Tools for Bio-decontamination.” Biotechnology Advances 26: 610-617.
[12] Niemira, B. 2012. “Cold Plasma Decontamination of Foods.” Annual Review of Food Science and Technology
3: 125-142.
[13] Tanarro, I., Herrero, V. J., Carrasco, E., and Jiménez-Redondo, M. 2011. “Cold Plasma chemistry and diagnostics.” Vacuum 85: 1120-1124.
[14] Bonizzoni, G., and Vassallo, E. 2002. “Plasma Physics and Technology; Industrial Applications.” Vacuum 64: 327-336.
[15] Knoerzer, K., Murphy, A., Fresewinkel, M., Sanguansri, P., and Coventry, J. 2012. “Evaluation of Methods for Determining Food Surface Temperature in the Presence of Low-Pressure Cool Plasma.” Innovative Food Science and Emerging Technologies 15: 23-30.
[16] Wan, J., Coventry, J., Swiergon, P., Sanguansri, P., and Versteeg, C. 2009. “Advances in Innovative Processing Technologies for Microbial Inactivation and Enhancement of Food Safety—Pulsed Electric Field and Low-Temperature Plasma.” Trends in Food Science & Technology 20: 414-424.
[17] Basaran, P., Basaran-Akgul, N., and Oksuz, L. 2008. “Elimination of Aspergillus parasiticus from Nut Surface with Low Pressure Cold Plasma (LPCP) Treatment.”
Food Micrbiology 25: 626-632.
[18] Gurol, C., Ekinci, F., Aslan, N., and Korachi, M. 2012. “Low Temperature Plasma for Decontamination of E. coli in Milk.” International Journal of Food Microbiology 157: 1-5.
[19] Noriega, E., Shama, G., Laca, A., Díaz, M., and Kong, M. 2011. “Cold Atmospheric Gas Plasma Disinfection of Chicken Meat and Chicken Skin Contaminated with Listeria innocua.” Food microbiology 28: 1293-1300.
[20] Bogaerts, A., Neyts, E., Gijbels, R., and Mullen, J. 2002. “Gas Discharge Plasma and Their Applications.”
Spectrochimica Acta Part B 57: 609-658.
[21] Hołub, M., Kalisiak, S., and Jakubowski, T. 2010. “Źródła Plazmy Nietermicznej Dla Technologii Ochrony
Środowiska.” Zeszyty Naukowe Wydziału Elektrotechniki i Automatyki Politechniki Gdańskiej 27: 1-4.
[22] Selcuk, M., Oksuz, L., and Basaran, P. 2008. “Decontamination of Grains and Legumes Infected with
Aspergillus spp. and Penicillum spp. by Cold Plasma Treatment.” Bioresource Technology 99: 5104-5109. [23] ICF Consulting. 2002. U.S. Environmental Protection
Agency Office of Air and Radiation Global Programs Division. “Byproducts of Sulfur Hexafluoride (SF6) Use in the Electric Power Industry.”
[24] Kim, J., Lee, D., and Min, S. 2014. “Microbial Decontamination of Red Pepper Powder by Cold Plasma.”
Food Microbiology 38: 128-136.
[25] Mazerski, J. 2009. “Analiza Podobieństwa.” In
doi: 10.17265/1934-7391/2014.12.003
Exopolysaccharides from Lactic acid Bacteria as
Corrosion Inhibitors
Ignatova-Ivanova Tsveteslava and Radoslav Ivanov
Department of Biology, University of Shumen, Shumen 9712, Bulgaria
Received: November 27, 2014 / Accepted: December 12, 2014 / Published: December 30, 2014.
Abstract: Bacterial EPSs (exopolysaccharides) are believed to play an important role in the environment by promoting survival strategies such as bacterial attachment to surfaces and nutrient trapping, which facilitate processes of biofilm formation and development. These microbial biofilms have been implicated in corrosion of metals, bacterial attachment to prosthetic devices, fouling of heat exchange surfaces, toxicant immobilization, and fouling of ship hulls. In this paper, data on EPS production and the effect of EPS on corrosion of steel produced by Lactobacillus fermentum Ts are presented and discussed. The Lactobacillus fermentum Ts strain was isolated from types of Bulgarian rye flour. It was tested for its ability to produce exopolysaccharides when cultivated in a media containing 10% sucrose, 10% fructose and 10% maltose. The study of the corrosive stability of steel samples was conducted on the gravimetrique method. The rate of corrosion, the degree of protection, and coefficient of protection have been calculated. The structure of layer over steel plates was analysed by SEM (scanning electron microscopy) JSM 5510. It could be underlined that 10% sucrose and 10% maltose in the media stimulated the process of protection of corrosion.
Key words: Corrosion, inhibitor, lactic acid bacteria, SEM.
I. Introduction
EPS (exopolysaccharide) is a term first used by Sutherland [1] to describe high-molecular-weight carbohydrate polymers produced by marine bacteria. EPSs can be found as in capsular material or as dispersed slime in the surrounding environment with no obvious association to any one particular cell [2]. Considerable progress has been made in discovering and developing new microbial EPSs that possess novel industrial significance [3]. A vast number of microbial EPSs were reported over the last decades, and their composition, structure, biosynthesis and functional properties have been extensively studied. In recent years the increased demand for natural polymers for pharmaceutical, food, and other industrial applications has led to a remarkable interest
Corresponding author: Ignatova-Ivanova Tsveteslava, Ph.D., associate professor, research fields: lactic acid bacteria as probiotics products, prebiotics—utilization of lactic acid bacteria, exopolysaccharides, corrosion, isolating microorganisms from Antarctica. E-mail: [email protected].
in polysaccharides produced by microorganisms. The physiological role of EPS depends on the ecological niches and the natural environment in which microorganisms have been isolated.
Bacterial EPSs (exopolysaccharides) are believed to play an important role in the environment by promoting survival strategies such as bacterial attachment to surfaces and nutrient trapping, which facilitate processes of biofilm formation and development [4]. These microbial biofilms have been implicated in corrosion of metals, bacterial attachment to prosthetic devices, fouling of heat exchange surfaces, toxicant immobilization, and fouling of ship hulls [5-7].
Corrosion of metals is a serious and challenging problem faced worldwide by industry. It has been estimated that the yearly corrosion damage costs are currently equivalent to 4.2% of the U.S. gross national product. These costs could be greatly reduced by better and wider use of corrosion protection techniques. Traditional methods of corrosion protection involve
D
the use of organic coatings to protect metal surfaces through barrier and passivation mechanisms. However, these coatings are not permanent and the cost of applying organic coatings on corroding components in use is extremely prohibitive. Applying coatings before the components are introduced into service involves excessive costs because they are susceptible to abrasions and other forms of mechanically induced damage. Thus, a coating that can be easily applied and maintained on corroding parts and is cost-effective is an attractive alternative to the prevention methods currently in use. Since bacteria can coat metals with a regenerative biofilm, it is becoming evident that they may be used as a means of preventing corrosion [8].
In this paper, data on EPS production and the effect of EPS on corrosion of steel produced by Lactobacillus fermentum Ts are presented and discussed.
2. Materials and Methods
2.1 Strain
Strain Lactobacillus fermentum Ts was obtained from the collection of the Department of Biology, Shumen University. Molecular analysis in LAB (lactic acid bacteria) was performed by molecular identification (16S rRNA gene sequencing) in GeXP Genetic Analysis System (Beckman Coulter, USA) [9].
2.2 Media
The strain cultivated in media of MRS (de Mann Rogosa Sharpe, Biolife 272-20128, Milano, Italia) in composition, g/L: Tween 80—1; pepton from casein—10.0; meat extract—8.0; yeast extract—4.0; K2HPO4—2.0; sodium acetat—5.0; amonium
citrate—2.0; MgSO4·7H2O—0.2 and MnSO4—0.05.
The pH of media was adjusted to 6.5 with 1 M NaOH. The basic media was sterilized by autoclaving at 121 °C for 20 min, and carbohydrates supplemented were sterilized using 0.22 µM filters (Manisart®). The basic MRS broth was supplemented with 10% sucrose; 10% fructose and 10% maltose to be tested.
2.3 Study of the Corrosive Stability
The study of the corrosive stability of steel samples was conducted with the gravimetrique method [10]. Before use, steel panels ( 10 × 4 × 0.2 mM) were treated with 70% C2H5OH, washed with water and
dried in an oven, cooled in a desiccator, weighed on a balance and kept in a desiccator unit used. The weight of the samples was measured using analytical balances. The dimensions of the samples were measured with micrometer. Three types of experimental series were performed:
(a) cultivation of the studied strain in mMRS media with 10 % of sucrose;
(b) in mMRS media with 10% fructose; (c) in mMRS media with 10% maltose.
Initially the steel samples were added in two variants: deproteinised supernatant and free cell supernatant. Then the steel samples were added in seawater as control probe and a dilution (3: 100) of the cultural media of the studied strain was added as inhibitor of the corrosion. The duration of the procedure was 120 h at 18 °C. After the treatment the steel samples were washed with water and dried to constant weight.
The structure of layer over steel plates was analised by SEM (scanning electron microscopy) JSM 5510.
2.4 Parameters of Corrosion
After retrieval, the corrosion products were removed when washed with water. They were dried in an oven. After the removal of corrosion, steel plates were cleaned and reweighed as above to estimate weight loss.
The rate of corrosion, the degree of protection, and coefficient of protection were calculated.
The corrosion rate K (g/cm2·h) was presented as follows:
In order to track out the inhibitor properties of EPS synthesized in media, the degree of protection (Z) and coefficient of protection (γ) have been calculated using the formulas:
Z = (K0 – Ki) / K0 × 100, % (2)
γ = K0 / Ki (3)
Where, K0 is the corrosion rate in control media;
Ki—the corrosion rate in test media
3. Results and Discussion
The corrosion of iron and its alloys causes severe economical loss resulting in a yearly cost of billions of dollars or euros. The use of heavy metals and heavy metal containing compounds, such as chromate, has to be reduced in coatings for some are known to be very toxic, even carcinogenic, and cause great environmental damage. Prevention of or reduction in the rate of corrosion may be accomplished by the use of a biological, environmentally friendly anti corrosive layer at the metal interface. The presence of EPS associated with bacterial cells can be recognized by the formation of colonies in mucous solid medium [11]. Therefore, the presence of a translucent or creamy material involving a mucoid colony is indicative of EPS production potential. When cultivated in a media with high content of saccharides such as 10% sucrose solutions, 10% fructose solutions, and 10% maltose solutions, strain L. fermentum Ts synthesizes exopolysaccharides (Fig. 1).
Fig. 1 EPSs (exopolysaccharides) produced by L. fermentum Ts cultivated in a media containing 10% sucrose,
which are secreted in the culture medium.
Similar experiments have also been demonstrated by other authors [12, 13].
Homopolysaccharides produced by GRAS (Generally Recognised as Safe) lactic acid bacteria are often synthesised by a single extra-cellular sucrase enzyme, using only sucrose as substrate [13]. They can be produced in largest quantities (bulk scale). Moreover, their structure can be modified allowing optimisation of their physicochemical properties. By means of cyclic voltammetry, impedance measurements and potential monitoring the electrochemical behaviour of a new type of anti-corrosive biopolymers has been studied, which can be deposited upon metal surfaces as layers. Besides this electrochemical characterisation, electrochemical measurements were used to select optimal biologically manufactured and chemically modified polymers.
Strain L. fermentum Ts was cultivated in a media containing 10% sucrose, 10% fructose, and 10% maltose for 12 h. The steel samples were placed in seawater as control probe and a dilution (3: 100) of the cultural media of the studied strain was added as inhibitor of the corrosion. The received results are presented in Table 1.
In our previous studies [14-17], it was shown that at the presence of high concentration of lactose (5% to 15%), high concentration of sucrose 4%, mixed sucrose 4% and 2% maltose and mixed sucrose 5% and 5% maltose, mixed 5% sucrose and 5% fructose and mixed 5% sucrose and 5% fructose the strains
Lactobacillus delbrueckii B5, L. delbrueckii K27, L. delbrueckii B8, L. delbrueckii O43, L. delbrueckii K3,
L. delbrueckii K17, and L. delbrueckii K15
synthesized exopolysaccharides which have inhibitory properties. It is well known that some lactobacillus strains such as genus Leuconostoc secreted trans glucosidases after cultivation in the presence of sucrose. The structure of the layer over the steel plates was analyzed by Scanning electron microscopy.
Table 1 Characterization of the protective properties in seawater with added supernatant.
No
sample Media
The quantity of the
supernatant in seawater, % K × 10 -5
, g/cm2·h Z, % γ
1 10% sucrose* 3.0 0.21 74.07 3.86
2 10% maltose 3.0 0.25 69.14 3.24
3 10% fructose 3.0 0.71 12.35 1.14
4 control 0.81 - -
*The steel plates were photographed after washing; results are mean ± SEM of three separate trails.
(a) (b)
Fig. 2 Biofilm formed by L. delbrueckii B5 on the surface of mild steel, visualized using SEM. (a) Steel plates after corrosion in seawater with inhibitor supernatant obtained of mixed 10% sucrose; (b) control—steel plates after corrosion in seawater.
The biofilm makes it not easily corrodible in seawater, supplemented with cultivated ambient from the same strain grown in a composite of 10% sucrose (Fig. 2a). Fig. 2b shows a picture of a steel surface sample treated directly with seawater. The observed lamellaes are most probably FeCl2 crystals, product of
the corrosion.
Microscope techniques provide information about the morphology of microbial cells and colonies, their distribution on the surface, the presence of EPS (Fig. 2a) and the nature of corrosion products (crystalline or amorphous; Fig. 2b). They can also reveal the type of attack (e.g., pitting or uniform corrosion) by visualizing changes in microstructure and surface features after removal of the biofilm and corrosion products (Fig. 2b).
The ability of EPS to bind specific metal ions strongly influences its adhesion to metal surface and its ability to concentrate metal ions from surfaces and bulk media. Binding of metals may be important in both passivation and activation reactions. The observed inverse relationship between EPS and the corrosion rates of mild steel suggests that similar reactions may be occurring in the natural environment leading to the formation of a protective film on the metal surface.
The corrosion of mild steel starts with generation of ferrous ions by anodic oxidation at the surface because of the reaction (Fe → Fe+2 + 2e- ) which may undergo further oxidation producing Fe+3 species (Fe+2
→ Fe+3 + e-). Ferric ions are particularly deleterious for mild steel as they tend to accelerate corrosion by the reaction (Fe+2 → Fe+3 + e-). If ferric ions are immobilized then it may be possible to control the corrosion of mild steel. Some polysaccharides are reported to exhibit the strongest stability constant for Fe3+ ions [18]. Such a complex may serve as a corrosion inhibitor. The observed inverse relationship between EPS and the corrosion rate of mild steel suggests that such a metal-polysaccharide complex was probably involved in developing a protective film on the metal surface in natural sea water.
The data suggest that biofilm EPS inhibits the corrosion of mild steel in natural marine waters.
4. Conclusions
From the received results it was evident that a mixture of 10% sucrose, or 10% maltose stimulated the formation of microbial biofilm inhibiting the corrosion of steel. The present research confirms the result of the pilot project [2] that polysaccharides made by microorganisms show anti-corrosive properties. Especially, homopolysaccharides showed interesting results for the protection of steel. Measurements indicate that it takes some time for layers of biopolymers on the metal to build a complete protective layer. The data showed that L. fermentum Ts produce EPS, which serve as corrosion inhibitor for mild steel.
Further studies are needed to evaluate the potential of the biofilm exopolysaccharides as anticorrosive agents.
Acknowledgments
The authors would like to express our gratitude for the support of this work to research grant FSI 08-213/10.03.2014 of Shumen University.
References
[1] Arrage, A. A., Vasishtha, N., Sundberg, D., Bausch, G., Vincent, H. L., and White, D. C. 1995. “On-Line Monitoring of Antifouling and Fouling-Release Surfaces Using Bioluminescence and Fluorescence Measurements during Laminar-Flow.” Journal of Industrial Microbiology 15: 277-282.
[2] Breur, H. J. A. 2001. “Fouling and Bioprotection of Metals: Monitoring and Control of Deposition Processes in Aqueous Environments.” Ph.D. thesis, Technische Universiteit Delft.
[3] Christensen, B. E., and Characklis, W. G. 1990. Physical and chemical properties of biofilms. New York: John Wiley & Sons.
[4] Costerton, W. J., Cheng, K. J., Geesey, G. G., Ladd, T. I., Nickel, J. C., Dasgupta, M., and Marrie, T. J. 1987. “Bacterial Biofilms in Nature and Disease.” Annual Review of Microbiology 41: 435-464.
[5] Ford, T. E., Maki, J. S., and Mitchell, R. 1988. “Involvement of Bacterial Exopolymers in Biodeterioration.” Biodeterioration 7: 378-384.
[6] Gómez, J. 2006. “Caracterización de los Exopolisacaridos Producidos por Microorganismo Shalófilos Pertenecientes a los Géneros Halomonas, Alteromonas, Idiomarina, Palleronia y Salipiger.” Ph.D. thesis, Universidad de Granada.
[7] Ignatova-Ivanova, Ts., Ananieva, M., Ivanov, R., Iliev, I., and Ivanova, I. 2014. “Biodiversity of Lactic Acid Bacteria in Bulgarian Wheat and Rye Flour.” Journal of BioScience and Biotechnology 101-105.
[8] Ignatova-Ivanova Ts., Ivanov, R., Iliev, I., and Ivanova, I. 2009. “Study Anticorrosion Effect of EPS from Now Strains Lactobacillus Delbruecii.” Biotechnol & Biotechnol EQ Special edition/on line 705-708.
[9] Ignatova-Ivanova, Ts., Ivanov, R., Iliev, I., and Ivanova, I. 2011. “Study of Anticorrosion Effect of Exopolysaccharides Produced Lactobacillus Delbrueckii b5 Cultivated on Different Carbohydrates.” Biotechnol & Biotechnol EQ Special edition/on line 224-227.
[10] Ignatova-Ivanova, Ts., and Ivanov, R., 2013. “Anticorrosion Effect of Biofilm Forming by Lactobacillus Strains on Metal Surfaces.” Bulgarian Journal of Agricultural Science 19 (2): 83-85.
[11] Ignatova-Ivanova, Ts. V., and Ivanov, R. I. 2014. “Study of Biofilm Formed by Lactic Acid Bacteria on the Surface of Mild Steel.” Journal of Life Sciences 8: 799-804. doi: 10.17265/1934-7391.
[13] Marshall, K. C. 1992. “Biofilms: an Overview of Bacterial Adhesion, Activity, and Control at Surfaces.”
ASM News 58: 202-207.
[14] Nicolaus, B., Kambourova, M., and Oner, E. T. 2010. “Exopolysaccharides from Extremophiles: from Fundamentals to Biotechnology.” Environmental Technology 31 (10): 1145-1158.
[15] Raychev, R., Fachikov, L. and Zaprjanova, V. 2002. “Corrosion and Protection of the Materials—Handbook
for Laboratorial Exercises.” Sofia.
[16] Sutherland, W. 1972. “Bacterial Exopolysaccharides.”
Advances in Microbial Physiology 8: 143-213.
[17] Sutherland, W. 1982. “Biosynthesis of Microbial Exopolysaccharides.” Advances in Microbial Physiology
23: 79-150.
doi: 10.17265/1934-7391/2014.12.004
Morphogenesis of Oil Palm (
Elaeis guineensis
Jacq.)
Fruit in Seed Development
Hermine Bille Ngalle1, Joseph Martin Bell1, Georges Franck Ngando-Ebongue2, Hernild Eman-Evina1, Godswill Ntsefong Ntsomboh2 and Armand Nsimi-Mva3
1. Department of Plant Biology, Faculty of Science, University of Yaoundé I, Yaoundé, P.O. Box 812, Cameroon 2. Specialized Oil Palm Research Centre (CEREPAH of La Dibamba), IRAD, Douala, P.O. 243, Cameroon 3. Ekona Regional Research Centre, IRAD, Buéa, P.O. Box 25, Cameroon
Received: November 14, 2014 / Accepted: December 2, 2014 / Published: December 30, 2014.
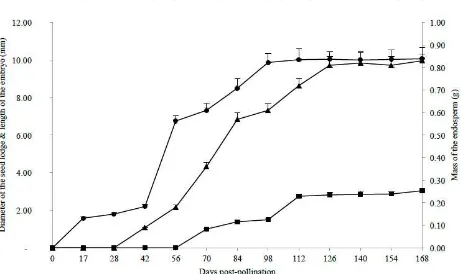
Abstract: The place of the oil palm, Elaeis guineensis Jacq., in the market for fats of vegetable commodities makes it a strategic plant which requires continuous improvement. In this context, it seems appropriate to better describe the effects of the Sh gene in the developing fruit. This study aims to set a benchmark for the development of the seed in the natural palm (Elaeis guineensis var. dura). Thus the growth and development of the two major seed tissues were monitored every two weeks from pollination to maturity of the fruit. The results show that the endosperm is still liquid six weeks after pollination. It then begins an accelerated development which leads it, 11 weeks later, to completely fill the seed cavity, with an average mass of 0.81 g. This mass remains stable until the maturity of the fruit. The embryo is only visible when the endosperm is gelatinous, around 70 DPP (days post-pollination). It then has an average length of 1.00 mm. At 126 DPP, the embryo has finished growing and measures 2.82 mm on average. This length also remains stable until 168 DPP (3.04 mm). In perspective, a detailed follow-up of the development of the zygote from the pollination to 100 DPP is proposed. In parallel, the analysis of the chemical composition of the endosperm between 100 DPP and 168 DPP is necessary. These two complementary studies will allow to better specifying the benchmark of seed development in Elaeis guineensis
var. dura.
Key words: Elaeis guineensis Jacq., embryo, endosperm, seed, development.
1. Introduction
Since 2006, palm oil, extracted from the mesocarp of the fruit of the oil palm (Elaeis guineensis Jacq.), became the first source of vegetable fat on the world market [1]. With a world production of 57.3 million tons in 2013 [2], this oil also ranks first in terms of production. Palm oil reaches this performance thanks to its exceptional yield, with world average around four tons of palm oil per hectare [3]. This productivity of the oil palm is much greater than that of all oilseed crops. It is ten times higher than that of soybean [3-5]. With a production of 6.8 million tons in 2013 [2], palm kernel oil extracted from the seed also holds an important
Corresponding author: Joseph Martin Bell, Ph.D., associate professor, research fields: genetics and plant breeding. E-mail: [email protected].
place in the consumption of fats of vegetable origin. Oil palm appears as a strategic plant for the economy of numerous producing countries.
Breeding programs and genetic improvement of this species are primarily focused on the development of planting material more efficient in terms of production of palm oil and kernels [6]. However, the history of selection in this plant is of recent [4, 7-10]. It consisted up to here in indirectly valuing a natural mutation that occurred on the shell (Sh) gene, which specifically controls the thickness of the endocarp in this species [11, 12]. At this locus, the wild palm dura having the genotype Sh+Sh+
with thick endocarp and large seed is distinguished from the mutated palm named pisifera having the genotype Sh-Sh- without endocarp and with a tiny seed, and the hybrid palm
D
tenera descended from the cross [♀dura × ♂pisifera], having the genotype Sh+Sh- with thin endocarp and normal seed [13, 14].
The Sh gene seems to have a direct impact on the endocarp and an indirect effect on the survival of the seed and thus on the female infertility in E. guineensis [6, 15]. This pleiotropy of the Sh gene has not yet been clarified. A very interesting orientation and a source of significant progress would be to develop a
pisifera planting material thus producing fruits without endocarp, but in which the indirect effect of the Sh gene mentioned above would be reduced or eliminated. In other words, it would be a matter to identifying candidate genes for the restoration of female fertility in E. guineensis Jacq. var. pisifera.
To better analyse the pleiotropy of this gene, it is necessary to have a precise benchmark for the development of oil palm fruit at a time when the mutated allele is not present. A recent study has already described the development of the pericarp of the fruit of the oil palm [16]. The general objective of the present work is to describe the development of the seed of E. guineensis Jacq. var. dura. Specifically, the study assesses changes in the seed lodge; determines the deadlines of appearance of the endosperm and the embryo, as well as the pace of development of these two tissues, from pollination to the fruit maturity.
2. Materials and Methods
The plant material is freely obtained from the CEREPAH (Specialized Oil Palm Research Centre) of La Dibamba, one of the stations of the IRAD (Institute of Agricultural Research for Development) in Cameroon. It consists of fruits collected from maturing oil palm bunches.
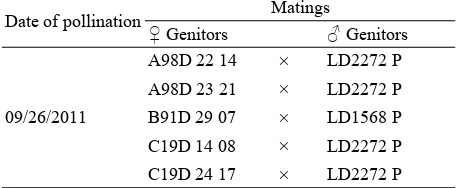
Five assisted pollinations were made between dura (female parent) and pisifera genitors (Table 1).
Fruits were sampled on bunches, every two weeks from the first DPP (day post-pollination) to the maturity of bunches. Maturity is substantiated by the natural detachment of the first fruits. At each stage of
Table 1 Genitors used and controlled pollinations made at CEREPAH (Specialized Oil Palm Research Centre).
Date of pollination Matings
♀ Genitors ♂ Genitors
development, 30 fruits were taken from the whole bunch, that is 150 fruits for five bunches.
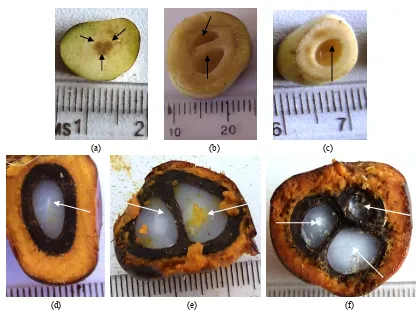
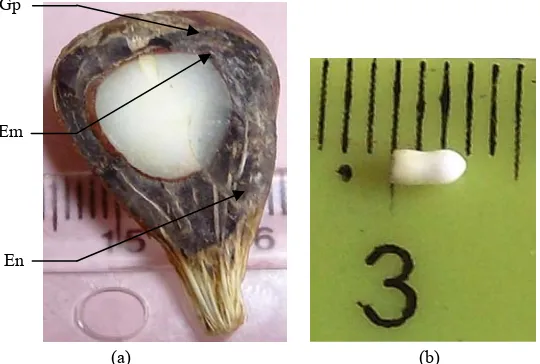
Fruits sampled underwent longitudinal or transverse sections, which were observed with the naked eye and/or by means of a EUROMAX optical microscope with a micrometre. These observations were essentially aimed at assessing the shape of the lodge of the seed, determining the time of onset of the endosperm and embryo. Different measurements on these tissues can track, from pollination fruit maturity, the evolution of the:
equatorial diameter of the lodge of the seed, measured microscopically for the early stages of fruit development (0-42 DPP). In later stages, this parameter is measured using a ruler;
consistency of the endosperm, appreciated with the naked eye and the touch;
mass of the endosperm. For young phases of fruit development (0-70 DPP), this parameter is estimated by the formula Men = Mwf - Mhf, where Men, Mwf and Mhf
represent respectively the masses of the endosperm, the whole fruit (with the seed) and the hollowed fruit (fruit freed of the seed). At the advanced stages, this parameter was measured using a precision balance 0.1 mg brand RADWAG (series AS/X), minimum and maximum capacity estimated at 10 mg and 220 g respectively;