www.elsevier.com / locate / bres
Research report
Intracerebroventricular injection of senktide-induced Fos expression in
vasopressin-containing hypothalamic neurons in the rat
Institute of Neurosciences, Fourth Military Medical University, Xi’an 710032, PR China
b
Department of Anatomy, Fourth Military Medical University, Xi’an 710032, PR China
c
Stomatological College, Fourth Military Medical University, Xi’an 710032, PR China
Accepted 8 August 2000
Abstract
Intracerebroventricular injection of senktide, a selective agonist for neurokinin B receptor (NK3), induced Fos expression in many neurons of the rat hypothalamus. Fos-positive neurons were predominantly present in the supraoptic and paraventricular hypothalamic nuclei, and some of them were seen in the lateral preoptic area, lateral hypothalamic area, arcuate nucleus, perifornical region, posterior hypothalamic area, circular nucleus, and along relatively large blood vessels (lateral hypothalamic perivascular nucleus) in the anterior hypothalamus. A double labeling study was performed to examine if vasopressin-containing neurons in the hypothalamus could be activated by the treatment. Neurons with both Fos-like immunoreactivity (-LI) and vasopressin-LI were found in the paraventricular nucleus, supraoptic nucleus, circular nucleus and lateral hypothalamic perivascular nucleus. In the supraoptic nucleus, about 87% of vasopressin-containing neurons exhibited Fos-LI, which corresponded to about 64% of Fos-positive neurons in the nucleus. In the paraventricular nucleus, about 80% of vasopressin-like immunoreactive neurons exhibited Fos-LI, which constituted about 51% of the total population of Fos-positive neurons in the region. The results suggest that NK3 receptor may be involved in the modulation of release of vasopressin from the hypothalamus in the rat. 2000 Elsevier Science B.V. All rights reserved.
Theme: Endocrine and autonomic regulation
Topic: Osmotic and thermal regulation
Keywords: Neurokinin B receptor; Senktide; Vasopressin; c-fos; Hypothalamus; Rat
1. Introduction (NK1), NKA receptor (NK2), and NKB receptor (NK3)
[20,23]. There have been numerous morphological, physio-The mammalian tachykinin family, which includes logical, and pharmacological studies on functions of SP substance P (SP) and neurokinin A (substance K; NKA) and its receptor, and a significant participation of SP/ NK1 and neurokinin B (neuromedin K; NKB), mediates diverse receptor in pain mechanisms is now well established biological responses, from a participation in sensory and [5,17]. However, much less is known about NK2 and NK3 motor functions to a role in the cardiovascular, respiratory, receptors, especially the latter, as specific antagonists have gastrointestinal, and immune systems and inflammation been developed for it only recently.
[25]. Their actions as neurotransmitters / neuromodulators Previous studies have suggested that NK3 receptor may are mediated by three distinct receptors termed SP receptor participate in some autonomic functions, such as osmotic regulation, ingestive behavior, and cardiovascular activity [3,4,7,12,22,25,27,28,31,36]. For example, intracerebro-*Corresponding author. Present address: Department of Cell Biology ventricular injection of senktide, a selective agonist for and Anatomy, University of North Carolina at Chapel Hill, CB[7090,
NK3 receptor, produced a potent, dose-dependent inhibi-Chapel Hill, NC 27599, USA. Tel.:11-919-966-1277; fax:1
1-919-966-tion of salt appetite and an increase in blood pressure and 1856.
E-mail address: yu-qiang [email protected] (Y.-Q. Ding).] heart rate [21,22]. The increase in blood pressure could be
inhibited by intravenous injection of vasopressin antago- Cruz Biotechnology, Santa Cruz, CA, USA) in PBS nist, suggesting that senktide-induced pressor response was containing 1% normal donkey serum (NDS), 0.3% Triton mediated by release of vasopressin [12,28]. Moreover, X-100 and 0.03% NaN3 overnight at room temperature. immunohistochemical and in situ hybridization studies The sections were then washed in PBS and incubated with have revealed that NK3 receptor is widely distributed in biotinylated goat anti-rabbit IgG (1:200; Vector Laborator-the central nervous system of Laborator-the rat especially in Laborator-the ies, Burlingame, CA, USA) in the NDS incubation medium hypothalamus [9,15], and that a large proportion of NK3 for 3–4 h, washed again, and reacted with an Elite ABC receptor-expressing neurons in the paraventricular and Kit (1:200; Vector) in PBS for 2–3 h. The sections were supraoptic nuclei of the hypothalamus contain vasopressin finally reacted with 0.02% diaminobenzidine and 0.003% [10]. However, the role of NK3 receptor in the modulation hydrogen peroxide in 0.05 M Tris–HCl buffer (pH 7.6) of release of vasopressin from the hypothalamus is not with nickel intensification.
fully understood. Therefore, in the present study,
experi-ments were designed to examine the effects of intracere- 2.3. Double labeling for Fos and vasopressin broventricular administration of senktide on Fos expression
in immunohistologically identified vasopressin-containing Since injection of 0.5 mg of senktide-induced maximal neurons in the rat hypothalamus. Fos expression in the hypothalamus (see Section 3), one series of sections from the rats with injection of 0.5 mg senktide was subjected to double immunohistochemical
2. Materials and methods staining with anti-Fos and anti-vasopressin antibodies; this
made possible the evaluation of the number of vasopressin-2.1. Preparation of animals and tissue containing neurons activated by the treatment. The sections were first processed for Fos immunohistochemistry by the Twenty-two male rats (Wistar) weighing 200–250 g PAP method: they were incubated with rabbit anti-Fos were used in the present study. They were housed in a antibody (1:2000; Santa Cruz Biotechnology) in the NDS temperature-controlled room (22628C) on a 12:12-h incubation medium overnight and then with goat anti-(07:00–19:00 h) light–dark cycle, with food and water rabbit IgG (1:100; Vector) in the same medium for 6 h. freely available. All protocols used in the present study Finally, the sections were reacted with rabbit PAP-complex have been approved by the Committee of Animal Use for (1:100; Sigma) for 4 h. The bound peroxidase was Research and Education of the Fourth Military Medical visualized by incubation with 0.02% DAB and 0.003% University, China. The rats were anesthetized by intra- H O2 2 for 20–30 min with nickel intensification. After peritoneal injection of sodium pentobarbital (40 mg / kg several washes in PBS, the sections were immunostained body weight) and fitted by stereotaxic surgery with a for vasopressin by the ABC method. The sections were stainless-steel guide cannula aimed at the lateral ventricle incubated with mouse anti-vasopressin antibody (1:100; [26]. Five to 6 days after the operation, the rats were PS-41; Gift from Dr. H. Gainer [1,38]) in the NDS anesthetized in the same way and senktide (Sigma, St. incubation medium overnight. The specificity of this Louis, MO, USA) dissolved in 8 ml saline was injected antibody has been verified by various methods, and this into the lateral ventricle through a stainless-steel injector antibody could distinguish neurons containing vasopressin temporarily inserted into the guide cannula. The doses of from those with oxytocin [1,38]. After being washed in senktide were 0, 5 ng, 50 ng, 0.5mg and 5mg (n54 for 0, PBS, the sections were incubated with biotinylated donkey 5 ng, 50 ng and 5 mg, respectively; n56 for 0.5 mg). anti-mouse IgG (10 mg / ml; Jackson Immunochemicals, Ninety minutes later, the rats were perfused with 100 ml of West Grove, PA, USA) for 8 h at room temperature, and 0.01 M phosphate-buffered saline (PBS; pH 7.4), followed then were treated with the Elite ABC Kit (1:200; Vector) by 500 ml of 4% paraformaldehyde and 0.1% picric acid in for 4 h at room temperature. To discriminate vasopressin-0.1 M phosphate buffer (PB; pH 7.4). All the perfusions in like immunoreactivity (-LI) from Fos-LI, the bound per-the present study were carried out between 11:00 and oxidase for vasopressin-LI was visualized with 0.02% 12:00 h on the day the experiment was performed. The DAB and 0.003% hydrogen peroxide in Tris–HCl buffer brains were removed immediately and placed in 20% (pH 7.6) without nickel intensification. In the control sucrose in PB overnight at 48C. The brains were cut into experiments, when one of the primary antibodies was five series of 30-mm thick transverse sections on a freezing omitted or replaced with normal rabbit (for Fos
immuno-microtome. histochemistry) or mouse (for vasopressin
immunohisto-chemistry) IgG, no immunoreactivity was detected in the
2.2. Fos immunohistochemistry control sections.
Fos was identified using the avidin–biotin immuno- 2.4. Quantitative analysis histochemical protocol. Briefly, one series of sections were
from the microscope in selected nuclei unilaterally in one 3. Results series of sections (12–14 sections; see Section 2.2) from
each rat; these serial sections covered whole extent of the 3.1. Fos expression in the hypothalamus paraventricular and supraoptic nuclei of the hypothalamus.
Data were expressed as mean6S.E.M. In the double In rats injected with 8 ml saline as control, Fos-like immunostaining experiment, the number of cells labeled immunoreactive neurons were present consistently in very for vasopressin, Fos, and double-labeled cells were low numbers in several ares of the hypothalamus. These counted in one series of sections (12–14 sections; see areas included the lateral preoptic area, paraventricular Section 2.3) in each of six rats, and the percentages of hypothalamic nucleus, and supraoptic nucleus (Fig. 3). double-labeled neurons from vasopressin- and Fos-positive In contrast, injection of senktide into the lateral ventricle neurons in the hypothalamus (paraventricular nucleus, resulted in an obvious increase of Fos expression in the supraoptic nucleus, circular nucleus, and lateral hypo- hypothalamus (Figs. 1–3). Fos-positive neurons were thalamic perivascular nucleus) were calculated for each rat, present with a high density in the medial and lateral
and then averaged. magnocellular parts of the paraventricular nucleus and the
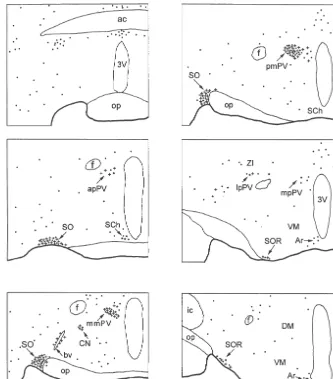
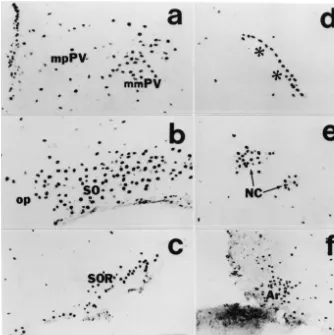
Fig. 2. Photomicrographs showing distribution of Fos-positive neurons in the paraventricular nucleus (a), principal part of the supraoptic nucleus (SO) (b), retrochiasmatic supraoptic nucleus (SOR) (c), lateral hypothalamic perivascular nucleus (d), circular nucleus (NC) (e), and arcuate nucleus (Ar) (f) of the hypothalamus. Rat was sacrificed 90 min after intracerebroventricular injection of 0.5mg of senktide. Asterisks indicate a relatively large blood vessel in the lateral anterior hypothalamus. mpPV, medial parvicellular part of the paraventricular nucleus. Bar560mm.
principal part of the supraoptic nucleus. With a lower In addition, many cells in the ependyma of the lateral density they were seen in the retrochiasmatic part of the and third ventricles and choroid plexus showed Fos-LI supraoptic nucleus, arcuate nucleus and circular nucleus. A after the intraventricular injections of senktide but not after few Fos-positive neurons were seen in the lateral preoptic injection of saline (Fig. 2a).
area, suprachiasmatic nucleus, anterior hypothalamic area, anterior magnocellular part of the paraventricular nucleus,
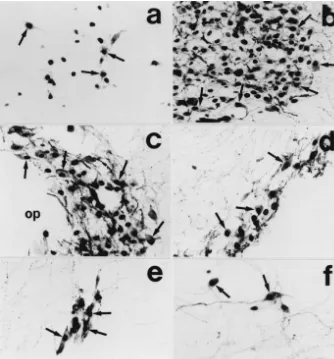
parvicellular part of the paraventricular nucleus, periforni- 3.2. Double labeling cal region, posterior hypothalamic area, and along
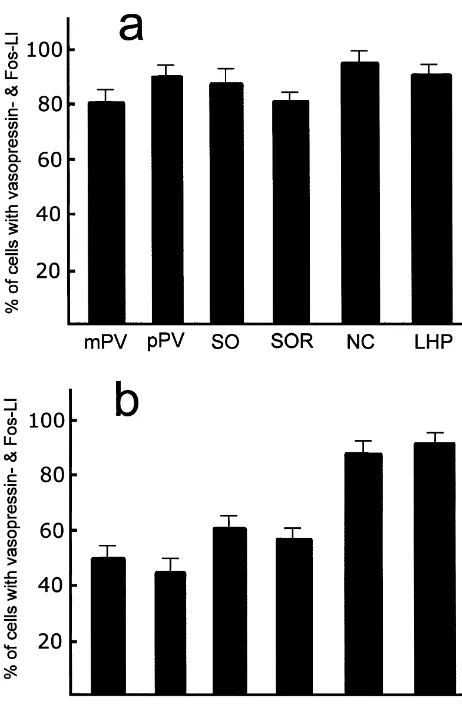
nucleus and lateral hypothalamic perivascular nucleus were 76, 84 and 90%, respectively (Fig. 5).
4. Discussion
The present study revealed that intracerebroventricular administration of senktide, a selective agonist for NK3 receptor, induced Fos expression in the hypothalamus, especially in the medial and lateral magnocellular parts of the paraventricular nucleus and in the supraoptic nucleus where vasopressin-containing neurons are abundant. Doub-le-labeling experiments indicated that the majority of the vasopressin-containing neuron in these regions exhibited Fos-LI.
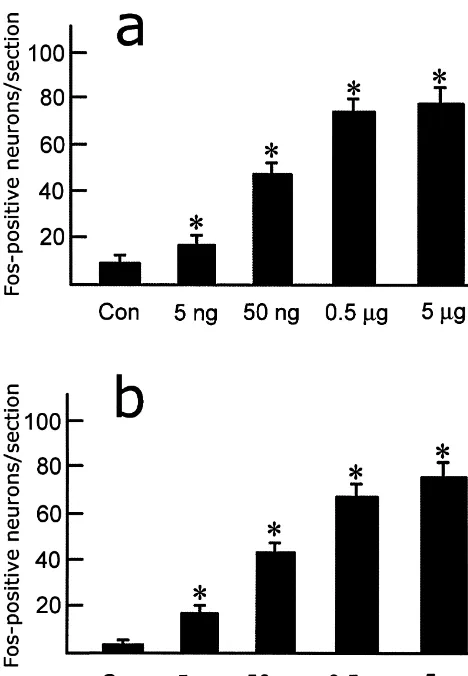
NK3 receptor-expressing neurons are distributed, with a high density, in the medial and lateral magnocellular parts of the paraventricular nucleus, and supraoptic nucleus, and with moderate density in the retrochiasmatic part of the supraoptic nucleus, lateral hypothalamic area, perifornical region and posterior hypothalamic area. A few of them are located in the circular nucleus, lateral hypothalamic perivascular nucleus, anterior hypothalamic area, but the dorsomedial and ventromedial hypothalamic nuclei con-tained no NK3 receptor-expressing neurons [9,10]. In general, the distribution pattern of Fos-positive neurons was well consistent with that of NK3 receptor in the hypothalamus. Thus, it is likely that the Fos-positive neurons may be directly activated by senktide injected into Fig. 3. Cell counts of Fos-positive neurons induced by intracerebroven- the lateral ventricle. On the other hand, it cannot excluded tricular injection of different doses of senktide (5 ng, 50 ng, 0.5mg and 5
that some of them might have been triggered to express
mg) in the paraventricular hypothalamic nucleus (a) and principal part of
Fos by other senktide-activated neurons in the hypo-the supraoptic nucleus (b). Each column represents mean6S.E. of
Fos-thalamus and even in other brain regions, because there are positive neurons per section, unilaterally. *P,0.01 versus control
ani-mals. complicated fiber connections among these structures and
the presence of Fos-LI cannot be used per se to distinguish first-order from second- or higher-order neurons within a stimulated pathway or to determine the direction of of the supraoptic nucleus, circular nucleus, and lateral activity-dependent information transfer between neurons. hypothalamic perivascular nucleus (Figs. 1, 4). However, In addition, the present study showed that Fos expression no double-labeled neurons were detected in the anterior was detected in many ependyma cells in the lateral and magnocellular part of the paraventricular nucleus. In the third ventricles and choroid plexus. It has been revealed medial and lateral magnocellular parts of the paraventricu- that there is no NK3 receptor-LI or mRNA was found in lar nucleus, about 80% of vasopressin-like immunoreactive these cells [9,10]. One possible explanation for this neurons expressed Fos-LI, constituted about 51% of the discrepancy is that NK3 receptor subtype may be present total population of Fos-positive neurons in the region (Fig. in these cells. However, it is also likely that senktide could 5). In the principal part of the supraoptic nucleus, about activate other tachykinin receptors in this region.
in-Fig. 4. Photomicrographs showing distribution of Fos / vasopressin double-labeled neurons in the anterior parvicellular part of the paraventricular nucleus (a), lateral magnocellular part of the paraventricular nucleus (b), principal part of the supraoptic nucleus (c), retrochiasmatic supraoptic nucleus (d), circular nucleus (e) and lateral hypothalamic perivascular nucleus (f). The rat was sacrificed 90 min after intracerebroventricular injection of 0.5mg of senktide. Arrows indicate a part of neurons expressing both vasopressin-LI and Fos-LI. op, optic chiasm. Bar530mm.
jection of hypertonic saline [10]; intravenous injection of accessory magnocellular group (for review, see Ref. [11]). hypertonic saline can increase plasma vasopressin level The accessory magnocellular neurons exhibited both vas-[2]. In addition, intracranial injection of senktide could opressin-LI and NK3 receptor-LI, and expressed Fos-LI increase blood pressure, and this effect could be blocked following the hypertonic treatment [10,24]. The functions by administration of vasopressin antagonist [21,31]. In of these neuron is not clear, although it has been indicated fact, it has been shown that intracerebroventricular in- that they are sensitive to osmotic stimulation and play an jection of senktide could increase plasma vasopressin important role in water balance, functions shared by both levels [12,28,31]. Taken together, the above evidence paraventricular and supraoptic nuclei [8,13,16,19]. It has indicates that the effects of intracranial administration of also been reported that the circular nucleus and lateral senktide on increase of plasma vasopressin level may, if hypothalamic nucleus are associated with maintaining and not all, at least in a part be due to a direct activation of reversing neurogenic hypertension [6,32,37] and establish-NK3 receptor in the paraventricular and supraoptic nuclei ment of dominant / subordinate relationships [14]. How-of the hypothalamus. Further studies are needed to ex- ever, based on the findings obtained in our present and amine the exact role of NK3 receptor in the modulation of previous studies, it seems to be likely that NK3 receptor-release of vasopressin from the hypothalamus under con- expressing neurons in the two nuclei may also be involved ditions of disturbed environment, such as dehydration, in the modulation of release of vasopressin from the
hypovolemia and hemorrhage. hypothalamus.
vasopressin-some Fos-positive neurons without vasopressin-LI in the paraventricular and supraoptic nuclei may contain oxy-tocin. However, to our knowledge, there has been no report about the possible interaction of NK3 receptor with oxytocin.
Acknowledgements
The authors are grateful to Dr. H. Gainer for generous supply of monoclonal antiserum against vasopressin, to Yue-Ping Yuan for photographic assistance, and to J.G. Valtschanoff for critical reading of the manuscript. This work has been supported by grants from the National Nature Science Foundation of China ([39600045, 39970377 to Y.Q.D).
References
[1] Y. Ben-barak, J.T. Russell, M.H. Whitnall, K. Ozato, H. Gainer, Neurophysin in the hypothalamo-neurohypophysial system: I. Pro-duction and characterization of monoclonal antibodies, J. Neurosci. 5 (1985) 81–87.
[2] M.J. Brimble, R.E.J. Dyball, Characterization of the response of oxytocin and vasopressin-secreting neurons in the supraoptic nu-cleus to osmotic stimulation, J. Physiol. 271 (1977) 253–271. [3] M. Broccardo, G. Improta, A. Tabacco, Central tachykinin NK3
receptors in the inhibitory action on the rat colonic propulsion of a new tachykinin, PG-KII, Eur. J. Pharmacol. 376 (1999) 67–71. [4] F. Cantalamessae, G. de Caro, M. Massi, M. Perfumi, Possible
influence of tachykinins on body fluid homeostasis in the rat, J. Fig. 5. Percentages of Fos / vasopressin double-labeled neurons from Physiol. (Paris) 79 (1984) 524–530.
vasopressin-labeled (a) and in Fos-labeled neurons (b) in the magnocellu- [5] Y.Q. Cao, P.W. Mantyh, E.J. Carlson, A.M. Gillespie, C.J.H. Epstein, lar part (mPV) and parvicellular part (pPV) of the paraventricular A.I. Basbaum, Primary afferent tachykinins are required to ex-hypothalamic nucleus, principal part (SO) and retrochiasmatic part (SOR) perience moderate to intense pain, Nature 392 (1998) 390–394. of the supraoptic nucleus, circular nucleus (NC), and lateral hypothalamic [6] J. Ciriello, Contribution of forebrain mechanisms in the maintenance perivascular nucleus (LHP). Data are mean6S.E. of six rats. of deoxycorticosterone acetate-salt hypertension, Clin. Exp.
Hy-pertens. 10 (1998) 169–178.
[7] G. de Caro, M. Perfumi, M. Massi, Tachykinins and body fluid regulation, in: A.N. Epstein, A. Morrison (Eds.), Progress in LI after intracranial injection of senktide, and that a large
Psychobiology and Physiological Psychology, Vol. 13, Academic number of positive neurons were located outside the Press, New York, 1988, pp. 31–61.
regions containing vasopressin in the hypothalamus. This [8] J.M. Ding, W.C. Carver, L. Terracio, J. Buggy, Protooncogene c-fos raises the possibility that NK3 receptor in the hypo- and the regulation of vasopressin gene expression during
dehydra-tion, Mol. Brain Res. 21 (1994) 247–255. thalamus may exert some functions other than modulation
[9] Y.-Q. Ding, R. Shigemoto, M. Takada, H. Ohishi, S. Nakanishi, N. of release of vasopressin. In fact, pharmacological and
Mizuno, Localization of the neuromedin K receptor (NK3) in the physiological experiments have indicated that centrally central nervous system of the rat, J. Comp. Neurol. 364 (1996) administered NK3 receptor agonist can modulate ex- 290–310.
¨
perimental anxiety [30], colonic transit [3] and visceral [10] Y.-Q. Ding, B.-Z. Lu, Z.-L. Guan, D.-S. Wang, J.-Q. Xu, J.-H. Li, Neurokinin B receptor (NK3)-containing neurons in the paraven-nociception [18]. Therefore, some Fos-positive neurons
tricular and supraoptic nuclei of the hypothalamus synthesize revealed in the present study may contribute to these
vasopressin and express Fos following intravenous injection of effects, because the hypothalamus is an important site hypertonic saline, Neuroscience 91 (1999) 1077–1085.
associated with these activities. In addition, since oxytocin [11] X. Duan, G. Ju, The organization of chemically characterized is the other neurohypophyseal hormone abundant in the afferents to the perivascular neuronal groups of the hypothalamic magnocellular neurosecretory system in the rat, Brain Res. Bull. 46 paraventricular and supraoptic nuclei of the hypothalamus,
(1998) 409–415. and since the present study revealed that Fos expression
[13] V.S. Fenelon, D.T. Theodosis, D.A. Poulain, Fos synthesis in release induced by intracranial injection of eledoisin is mediated by identified magnocellular neurons varies with phenotype, stimulus, central angiotensin II, Pharmacol. Res. Commun. 20 (1988) 811– location in the hypothalamus and reproductive state, Brain Res. 662 826.
(1994) 165–177. [28] C. Polidori, A. Saija, M. Perfumi, G. Costa, G. de Caro, M. Massi, [14] C.F. Ferris, J.F. Axelson, A.M. Martin, L.F. Roberge, Vasopressin Vasopressin release induced by intracranial injection of tachykinins immunoreactivity in the anterior hypothalamus is altered during the is due to activation central neurokinin-3 receptors, Neurosci. Lett. establishment of dominant / subordinate relationships between ham- 103 (1989) 320–325.
sters, Neuroscience 29 (1989) 675–683. [29] C.H. Rhodes, J.I. Morrell, D.W. Pfaff, Immunohistochemical analy-[15] B. Griffond, P. Giofi, L. Bayer, C. Jacquemard, D. Fellmann, sis of magnocellular elements in rat hypothalamus: distribution and Immunocytochemical detection of the neurokinin B receptor (NK3) numbers of cells containing neurophysin, oxytocin and vasopressin, on melanin-concentrating hormone (MCH) neurons in the rat brain, J. Comp. Neurol. 198 (1981) 45–64.
J. Chem. Neuroanat. 12 (1997) 183–189. [30] S.J. Riberiro, R.M. Teixerira, J.B. Calixto, T.C. Lima, Tachykinin [16] G.I. Hatton, W.E. Armstrong, W.A. Gregory, Spontaneous and NK(3) receptor involvement in anxiety, Neuropeptides 33 (1999)
osmotically stimulated activity in slices of rat hypothalamus, Brain 181–188.
Res. Bull. 3 (1978) 497–508. [31] A. Saigo, Y. Takano, T. Matsumoto, M. Tran, Y. Nakayama, R. [17] J.L. Henry, Effects of substance P on functionally identified units in Saito, K. Yamada, H. Kamiya, Central administration of senktide, a cat spinal cord, Brain Res. 114 (1976) 439–452. tachykinin NK-3 agonist, has an antidiuretic action by stimulating [18] V. Julia, X. Su, L. Bueno, G.F. Gehart, Role of neurokinin 3 AVP release in water-located rats, Neurosci. Lett. 159 (1993) 187–
receptors on responses to colorectal distention in the rat: electro- 190.
physiological and behavioral studies, Gastroenterology 116 (1999) [32] J.K. Simon, T.X. Zhang, J. Ciriello, Renal denervation alters 1124–1131. forebrain hexokinase activity in neurogenic hypertensive rats, Am. J. [19] T.L. Krukoff, D.H. Vincent, Regional alterations in hexokinase Physiol. 256 (1989) R930–R938.
activity within rat brain during dehydration and rehydration, Am. J. [33] M.N. Sofroniew, Vasopressin, oxytocin and their related
neuro-¨ ¨
Physiol. 256 (1989) R1050–R1055. physins, in: A. Bjorklund, T. Hokfelt (Eds.), Handbook of Chemical [20] J.E. Maggio, Tachykinins, Annu. Rev. Neurosci. 11 (1988) 13–28. Neuroanatomy, Vol. 4, Part I, Elsevier, Amsterdam, 1985, pp. 93– [21] M. Massi, C. Polidori, L. Gentili, M. Perfumi, G. de Carlo, C.A. 165.
¨ ¨
Maggi, The tachykinin NH -senktide, a selective neurokinin B2 [34] L.W. Swanson, The hypothalamus, in: A. Bjorklund, T. Hokfelt, receptor agonist, is a very potent inhibitor of salt appetite in the rat, L.W. Swanson (Eds.), Handbook of Chemical Neuroanatomy, Vol. 5, Neurosci. Lett. 92 (1988) 341–346. Part I, Elsevier, Amsterdam, 1987, pp. 1–104.
[22] A. Nagashima, Y. Takano, K. Tateishi, Y. Matsuoka, T. Hamaoka, H. [35] L.W. Swanson, H.G.J.M. Kuypers, The paraventricular nucleus of Kamiya, Cardiovascular roles of tachykinin peptides in the nucleus the hypothalamus: cytoarchitecture subdivisions and organization of tractus solitarlii of rats, Brain Res. 487 (1989) 392–396. projections to the pituitary, dorsal vagal complex, and spinal cord as [23] S. Nakanishi, Mammalian tachykinin receptors, Annu. Rev. Neuro- demonstrated by retrograde fluorescence double-labeling methods, J.
sci. 14 (1991) 123–136. Comp. Neurol. 194 (1980) 555–570.
[24] B.J. Oldfield, R.J. Bicknell, R.M. McAllen, R.S. Weisinger, M.J. [36] Y. Takano, A. Nakashima, T. Hagio, K. Tateishi, H. Kamiya, Role McKinley, Intravenous hypertonic saline induces Fos immuno- of central tachykinin peptides in cardiovascular regulation in rats, reactivity in neurons throughout the lamina terminalis, Brain Res. Brain Res. 528 (1990) 231–237.
561 (1991) 151–156. [37] W.E. Turton, J. Ciriello, F.R. Calaresu, Changes in forebrain [25] M. Otsuka, K. Yoshioka, Neurotransmitter functions of mammalian hexokinase activity after aortic baroreceptor denervation, Am. J.
tachykinins, Physiol. Rev. 73 (1993) 229–308. Physiol. 251 (1986) R274–R281.