Journal of Life Sciences
Volume 6, Number 3, March 2012 (Serial Number 47)
David Publishing Company www.davidpublishing.com
Publication Information
Journal of Life Sciences is published monthly in hard copy (ISSN 1934-7391) and online (ISSN 1934-7405) by David Publishing Company located at 9460 TELSTAR AVE SUITE 5, EL MONTE, CA 91731, USA.
Aims and Scope
Journal of Life Sciences, a monthly professional academic journal, covers all sorts of researches on molecular biology, microbiology, botany, zoology, genetics, bioengineering, ecology, cytology, biochemistry, and biophysics, as well as other issues related to life sciences.
Editorial Board Members
Dr. Stefan Hershberger (USA), Dr. Suiyun Chen (China), Dr. Farzana Perveen (Pakistan), Dr. Francisco Torrens (Spain), Dr. Filipa João (Portugal), Dr. Masahiro Yoshida (Japan), Dr. Reyhan Erdogan (Turkey), Dr. Grzegorz Żurek (Poland), Dr. Ali Izadpanah (Canada), Dr. Barbara Wiewióra (Poland), Dr. Valery Lyubimov (Russia), Dr. Amanda de Moraes Narcizo (Brasil), Dr. Marinus Frederik Willem te Pas (The Netherlands), Dr. Anthony Luke Byrne (Australia), Dr. Xingjun Li (China), Dr. Stefania Staibano (Italy), Dr. Wenle Xia (USA), Hamed Khalilvandi-Behroozyar (Iran).
Manuscripts and correspondence are invited for publication. You can submit your papers via Web Submission, or E-mail to [email protected] or [email protected]. Submission guidelines and Web Submission system are available at http://www.davidpublishing.com.
Editorial Office
9460 TELSTAR AVE SUITE 5, EL MONTE, CA 91731, USA Tel: 1-323-9847526, Fax: 1-323-9847374
E-mail:[email protected], [email protected]
Copyright©2012 by David Publishing Company and individual contributors. All rights reserved. David Publishing Company holds the exclusive copyright of all the contents of this journal. In accordance with the international convention, no part of this journal may be reproduced or transmitted by any media or publishing organs (including various websites) without the written permission of the copyright holder. Otherwise, any conduct would be considered as the violation of the copyright. The contents of this journal are available for any citation. However, all the citations should be clearly indicated with the title of this journal, serial number and the name of the author.
Abstracted / Indexed in
Database of EBSCO, Massachusetts, USA Chemical Abstracts Service (CAS), USA Cambridge Scientific Abstracts (CSA), USA
Chinese Database of CEPS, American Federal Computer Library center (OCLC), USA Ulrich’s Periodicals Directory, USA
Chinese Scientific Journals Database, VIP Corporation, Chongqing, China
Subscription Information
Price (per year): Print $520, Online $360, Print and Online $680.
David Publishing Company
9460 TELSTAR AVE SUITE 5, EL MONTE, CA 91731, USA Tel: 1-323-9847526, Fax: 1-323-9847374
E-mail: [email protected]
David Publishing Company www.davidpublishing.com
J LS
Journal of Life Sciences
Volume 6, Number 3, March 2012 (Serial Number 47)
Contents
Microbiology and Biochemical Pharmacy
243 Phytochemical Screening and Antimicrobial Evaluation of the Aqueous Extracts of Ammoides verticillata, an Endemic Species
Oumessaad Toubal, Abdelghani Djahoudi, Chérifa Henchiri and Mohamed Bouazza
248 The Survey of Microbiological Contamination of Pitcher Cheese in West Azarbayjan Province,
Iran
Ehsan Barati, Mona Daneshgar Moghaddam, Navab Ghobadi, Hamid Reza Shafieian and Abbas Barin
253 Detection of “Candidatus Phytoplasma asteris” in Brussels Sprout and Its Possible Association with Flower Bud Failure in Poland
Maria Kamińska, Hanna Berniak and Piotr Kamiński
260 The Role of Heat Shock Protein 70, IgE and MMP-9 in Detecting Early Minor Myocardial
Damage and Evaluating the Efficacy of Coronary Artery Bypass Grafting (CABG)
Amal A. Baalash, Hala E. Hamouda, Ghada M. Ismail, Ibrahim K. Yassein and Bedir M. Ibrahim
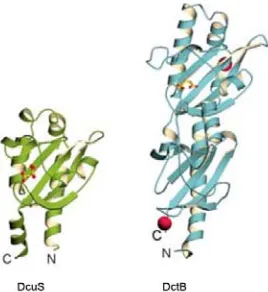
268 Molecular Cloning of PhoR Sensor Domain from Mycobacterium tuberculosis for Structure-Based Discovery of Novel Anti-Tubercular
Aldina S. Suwanto, Ihsanawati and Ernawati Arifin Giri-Rachman
Botany and Zoology
276 Effect of Cement Dust Pollution on the Yield and Quality of Ficus carica L. Fruits
Amal Mohamed Abdel-Rahman
283 The Effect of Drought Occurring at Different Growth Stages on Productivity of Grain Amaranth
Amaranthus cruentus G6
287 Structural Changes of Lignified Tissues from Sugarcane Leaves Induced by Smut (Sporisorium scitamineum) Virulence Factors
Borja Alarcón, Rocío Santiago, Carlos Vicente and María Estrella Legaz
300 The Contribution of the Dromedary in the Spontaneous Plant Seeds Transfer in the Northern
Algerian Sahara
Trabelsi Hafida, Senoussi Abdelhakim, Chehma Abdelmadjid and Faye Bernard
304 Biological Status of Captive Crane in Southern Districts of Northern Pakistan
Farzana Perveen
312 Effect of the Variety of Fig Tree on Some Biological Parameters of Ceratitis capitata Wied. 1824 (Diptera: Trypetidae) in Some Orchards in the Kabylie
Sadoudi-Ali Ahmed Djamila, Nabila Rezoug, Ferroudja Saiki and Noreddine Soltani
Interdisciplinary Researches
320 Community-Based Health Insurance: An Evolutionary Approach to Achieving Universal
Coverage in Low-Income Countries
Hong Wang and Nancy Pielemeier
330 Comparison of GFR by Creatinine Clearance with Estimated GFR by Various Prediction
Equations in a Bangladeshi Population
Muhammad Saiedullah, Muhammad Rezwanur Rahman, Md. Aminul Haque Khan, Shoma Hayat and Shahnaj Begum
335 The International Research Group in Geophysics Europe Africa: A Laboratory without Borders
in the Earth Science and Environment
Christine Amory-Mazaudier
342 The Effect of Anthropogenic Increase on the Earth as a Life-Support System for Mankind
Nickolay Pechurkin and Lydia Somova
348 A Note on the Current Status of Arin, a Yoruba Traditional Game Played with the Seeds of
Dioclea reflexa
Journal of Life Sciences 6 (2012) 243-247
Phytochemical Screening and Antimicrobial Evaluation
of the Aqueous Extracts of Ammoides verticillata, an
Endemic Species
Oumessaad Toubal1, Abdelghani Djahoudi2, Chérifa Henchiri3 and Mohamed Bouazza4
1. Department of Biology, Badji Mokhtar University, Annaba 23000, Algeria
2. Department of Pharmacy, Badji Mokhtar University, Annaba 23000, Algeria
3. Department of Biochemistry, Badji Mokhtar University, Annaba 23000, Algeria
4. Department of Botany, Aboubekr Belkaid University, Tlemcen 13000, Algeria
Received: February 10, 2012 / Accepted: February 29, 2012 / Published: March 30, 2012.
Abstract: Ammoidesverticillata (Desf.) Briq. is an Algerian endemic species; the phytochemical screening (methods of Harborne, 1973) of the aerials parts wealth in polyphenol compounds: flavonoids, saponins, leucoanthocyanes, terpens and steroids and tannins; there is no alkaloids. There is an important quantity of essential oils in the flowers; the interest of this study is that this species remains until then it is not very known. The results of Aromatogram method by incorporation of Müller-Hinton on solid medium, showed a significant antimicrobial activity (method of Duraffourd, 1987) of the infusion and the ethanolic extract; the infusion of stems and flowers is indeed much more active on Echerichiacoli, Citrobacter, Enterobacter, Staphaureus and Staphepidermidis, such as flowers extracts demonstrate an important antimicrobial activity on Staph aureus, Staph epidermidis, Streptococcus, Pseudomonas and Acinetobacter recognized as antibiotic resistant. This could give opportunities for using this species in the treatment of diverse infections and as a disinfecting additive on nosocomial area. The valorization, preservation and sustainable use of Ammoidesverticillata require the protection of its habitats.
Key words:Ammoidesverticillata, aqueous extracts, phytochemical screening, antimicrobial activity, disinfection.
1. Introduction
Ammoides verticillata (Desf.) Briq., a flowering grassy plant in the Apiaceae, is an endemic of northwestern Algeria and southern Mediterranean
Europe; this species was traditionally used for its culinary and medicinal properties (against flu,
intestinal parasites, as laxative and as poultice against boil) and it is often used as decoction and infusion; it is rich on thymol, a phenolic compound known for its
antimicrobial properties [1]. The antimicrobial activity of Ammoides verticillata (Av) essential oils was
studied by Abdelouahid and Bekhechi [2] who
Corresponding author: Oumessaad Toubal, Ph.D., professor, research fields: ecology and plant biotechnology. E-mail: [email protected].
demonstrated that essential oils are active on all the tested strains, but their activity is more important on mushrooms than on bacterias. Bekhechi et al. [3]
demonstrated that isothymol was the major component (51.2%) of this plant with y-cymene
(14.1%), thymol (13.0%), limonene (11.9%) and y-terpinene (6.8%); Av seed oil is known to be a rich source of natural thymol. The antifungal properties
against Aspergillusniger and Fusarium sp. studied by Chaker et al. [4]; whose test results showed that the
polar (hexane/dietyl ether 2/1) and non polar (hexane) extracts had moderate antifungal activity, and the Av
extracts were more efficient.
Phytochemical Screening and Antimicrobial Evaluation of the Aqueous
Extracts of Ammoides verticillata, an Endemic Species
244
activity against Aspergillusflavus (rate of inhibition = 100%) which seems to be related to a high content of
thymol; they found the highest yield of Av essential oils (3.09%).
The aim of this work is to study the phytochemical screening and the antimicrobial evaluation of the aerial parts (stems, flowers) of Av towards various
bacterial strains. This study wasn’t made before. A similar study was conducted on Genista numidica by
Toubal et al. [6]; recently, the acceptance of traditional medicine as an alternative to the available antibiotics has led authors to investigate the antimicrobial activity
of medicinal plants [7]. Moreover, the increasing use of plant extracts in food, cosmetic and pharmaceutical
industries suggests that, in order to find active compounds, a systematic study of medicinal plants is very important [8].
2. Materials and Methods
2.1 Plant Material
The plant was collected on April 2010 [9] at
Tlemcen Mountain (1,000 m) on calcareous soil [10]. Nomenclature is about its vernacular name which is Nunkha, Ajowan [11]. The harvesting, drying,
grinding, and chemical and microbial tests were conducted on May 2011.
2.2 Different Analysis
The microscopic analysis was made in Botany Laboratory, Faculty of Sciences, Annaba; a small quantity of powder is put down on the blade of
microscope with lactic reagent [12] and colored with iodine, covered with a small strip and warmed.
The chemical screening consisted on preliminary tests on the infusion (10%), searching for saponins, anthocyanes, tannins and leucoanthocyanes, and the
preliminary tests on the powder searching for alkaloids, flavonoids, terpenes and steroids; the notation is (+) for
a low presence, (++) for a middle presence, and (+++) for a high presence, all these tests were carried out according to Harborne [13] methods. We determined
also the proteins % by Kheldjal method.
The antimicrobial activity was made on raw plant extracts (10%) and infusion (10%) of stems and
flowers; the extraction was made by the soxlhet with ethanol solvents. These extracts are cleared of solvents
by evaporation (using a rotavapor) before incorporation against bacterial strains ATCC (American Type Culture Collection): gram+ and
gram-oxidative and fungus as follows: Escherichia coli, Klebsiella pneumonia, Proteus mirabilis,
Staphylococcus aureus, Pseudomonas aeruginosa,
Acinetobacter baumanii, Streptococcus faecalis,
Staphylococcus epidermidis, Proteus vulgaris,
Enterobacter aerogenes and Candida albicans which were obtained from the Bacteriology Laboratoy,
Faculty of Medicine, HCU of Dorban in Annaba. The antibacterial activities were carried out by Aromatogram by diffusion method of Müller-Hinton
on solid medium; the strains were reactivated using a 20 h culture growth at 37 °C and adjusted to 108
CFU/mL. The bacterial strains are sowed on the surface of the agar in radial spots form by means of swab and suspensions of young bacterial cultures
prepared according to the CLSI (committee for laboratory standards institute [14]). The application
is made by sterile filters paper disks (6 mm diameter, 06/limp) which were placed on the inoculated agar surfaces and impregnated with 10 µL of each
solution (10% dilution); the plates were incubated during 24 h at 37 °C [15]. The reading of the results
is made by the measurement of the inhibition diameter around the disk.
3. Results and Discussions
The aerial parts of the plant should be treated
separately because the antimicrobial activity is linked to the origin of the extract (stem, flower), the solvents and the used strains [16].
3.1 Microscopic Analysis
The originality of this study was to identify Av by
Phytochemical Screening and Antimicrobial Evaluation of the Aqueous
Extracts of Ammoides verticillata, an Endemic Species
245
recognizable to differentiate this plant species; representative elements of flowers and stems powder
of Av were observed; in the powder of flowers, there are abundant pollen grains with exine, intine and a
thickening of cellulose; there are also secretor canal of essential oils (Fig. 1a) which constitute a discriminate character allowing of family recognition.
3.2 Chemical Composition
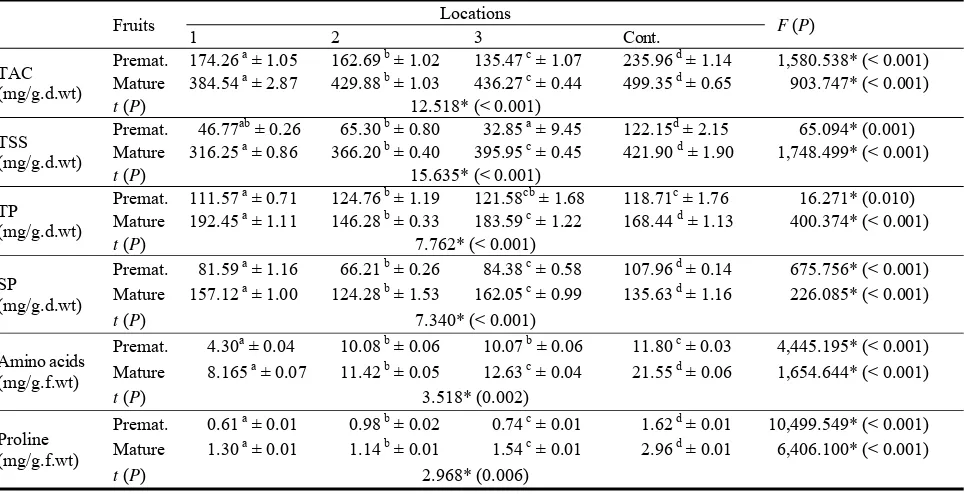
The phytochemical screening (Table 1) of Av
showed a wealth in polyphenolics compounds:
flavonoids, anthocyanes and tannins with a great quantity in stem and flowers; leucoanthocyanes don’t exist in flowers; saponins don’t exist in stem. There
are no alkaloids in the stem and flowers; terpenes and steroids exist in stems and flowers but with a little
quantity. The present investigation showed essential oils in the flowers.
3.3 Antimicrobial Activity
The various tests of sensitivity of Av demonstrate a
considerable antimicrobial activity of its diverse extracts towards tested strains; this antibacterial
evaluation was not reported previously; it is about cultures of bacteria gram+ and gram-oxidative and fungus cited before.
3.3.1 Sensitivity to the Flowers
The results are listed in Table 2; the extract
inhibition zone diameter is 8.3-18.7 mm; there is
a. Pollen grain, Gr: 60 × 10. b. Citrobacter freundii.
c. Staphylococcus epidermidis. d. Staphylococcus aureus. Fig. 1 Pollen grains (a) and bacterial inhibition zone diameter by flower infusion (b) and extract (c, d).
Table 1 Phytochemical screening of Av.
Components Stems Flowers
Flavonoids +++ ++
Saponins - +
Anthocyanes +++ ++
Terpenes & steroids ++ +
Tannins +++ +++
Leucoanthocyanes ++ -
Alkaloïdes - -
Table 2 Bacterial inhibition zone diameter (mm) by Av
flowers.
Bacterial strains Extract Infusion
Escherichia coli 8.3 9.2
Proteus vulgaris < 6 < 6 Citrobacter freundii < 6 11.9 Proteus mirabilis < 6 < 6
Klebsiella pneumoniae 9.1 < 6
Enterobacter aerogenes 9.7 9.7
Staphylococcus aureus 18.7 9.5
Staphylococcus epidermidis 17.2 10.5
Streptocoque faecalis 10.7 < 6
Pseudomonas aeruginosa 11.7 < 6
Acinetobacter bauannii 11.7 8.7
about 80% of sensitivity essentially for Staph. a
17.2 mm and Staph. ep. 18.7 mm (Fig. 1); Proteus v,
Proteus m and Citrobacter are resistant, with the
infusion inhibition zone diameter 8.7-11.9 mm,
Citrobacter and Staph ep are the most sensitive bacterias; efficiency is about 60%; Proteus m, Proteus
v, Pseudomonas a, and Streptococcus faecalis are the very resistant ones.
3.3.2 Sensitivity to the Stems
The extract inhibition zone diameter is ranged from
7.1 to 10.6 mm; extract is active only against E. coli
8.4 mm and Staphylococcus epidermidis 10.6 mm;
then there is about 70% of resistant strains, and the efficiency is about 30%; the infusion inhibition zone
diameter is 9.5-11.2 mm; the infusion act on E. coli,
Staph. ep, Enterobacter, Staph. aureus and Citrobacter, then its efficiency is about 50% (Fig. 2).
3.3.3 Comparison between Stems and Flowers Antimicrobial Activity
More relevant results were obtained with the
Phytochemical Screening and Antimicrobial Evaluation of the Aqueous
Extracts of Ammoides verticillata, an Endemic Species
246
0 2 4 6 8 10 12 14
Flowers infusion Stems infusion
Fig. 2 Bacterial inhibition zone diameter by Av stems and flowers infusion.
Fig. 3 Bacterial inhibition zone diameter by Av stems and flowers extract.
and showed the maximal activity 18.7 mm, within
the stems extract 11.2 mm act only on 30% of them
(Fig. 3). Proteus v and Proteus m seem to be the most
resistant bacteria within Klebsiella, Streptococcus and
Pseudomonas that have a medium resistance to all the
extracts. Stems and flowers infusion: a similar
antimicrobial activity excepted for Acinetobacter
which is sensible to flowers infusion but resistant to
stems one; then oxidative and fermentative gram– bacilli show an increase sensitivity (8.4-11.9 mm),
which seems to be significant, whereas gram + cocci
show a good sensitivity (7.1-18.7 mm) which doesn’t
seem to be significant.
4. Conclusions
A chemical and microbial studies were realized on
Av, an endemic species; chemical screening of its
aerials parts indicates a wealth in polyphenolics compounds; the results of aromatogram of raw plant extract as well as the infusion, showed a significant antimicrobial activity towards bacterial tested strains, especially bacilli gram of enterobacteriaceae recognized as antibiotic resistant, particularly in the case of urinary infections. It seems that it is the flower
extract which has more effect essentially on Staphyl.
aureus and Staphyl. epidermidis and the stem extract which has the low one. For the rest, flower extract and
I
nhibition zone diamete
r
(mm
)
20
15
10
5
0
strains
Inhibition zone dia
m
ete
r
(mm
Phytochemical Screening and Antimicrobial Evaluation of the Aqueous
Extracts of Ammoides verticillata, an Endemic Species
247
infusion seem to have a complementary effect.
This could give opportunities for using this species
in the treatment of urinary, respiratory, intestinal and cutaneous infections and as a disinfecting additive on nosocomial area. Flavonoids must be tested because
the antimicrobial activity is principally due to flavonoids and terpenes; free fatty acids can be
regarded as potential bactericidal [17, 18], then it will be interesting to test Av ones. The investigations to determine the degree of Av toxicity are in progress.
We also have to test the effect of different dilutions of the plant extracts and determine its inhibitory
minimum concentration.
All these results constitute only a first step in the research of natural substances biologically active;
complementary trials will be necessary to confirm the revealed performances, because several variables can
influence the results, such as the environmental and climatic conditions of the plant, the choice of the extraction methods and of the antimicrobial tests. The
valorization, preservation and sustainable use of Av
require the protection of its habitats.
Acknowledgments
This study was partly supported by MESRS
(Ministry of Scientific Research) of Algeria (Project CNEPRU, No. F-0112008001).
References
[1] K. Ettayebi, J.El. Yamani, B.D. Rossihasani, Synergistic effects of nisin and thymol on antimicrobial activities Listeria nonocytogenes and Bacillus subtilis, FEMS Microbial Lett. 183 (2000) 191-195.
[2] D.E. Abdelouahid, C. Bekhechi, Pouvoir antimicrobien de l’huile essentielle d’Ammoides verticillata (Nûnkha), Biol et Santé 4 (2) (2004) 1-10.
[3] C. Bekhechi, J.B. Boti, F.A. Bekkara, D.E. Abdelouahid, J. Casanova, F. Tomi, Isothymol in ajouan essential oil, Nat. Prod. Com. 5 (7) (2010) 1107-1110.
[4] A.N. Chaker, H. Laouer, M.M. Zerroug, Antifungal activity of three Apiaceae (Ammoides verticillata Desf.) Briq., Magydaris pastinaceae (Lamk.) Paol. and Bupleurumplantagineum Desf.) organic extracts, Revue des Régions Arides (Tunis) (2007) 420-422.
[5] Z. Mohammedi, S. Bachik, N. Bekaroube, Antifungal and
antiaflatoxinogenic potential of essential oils from an endemic thymus fontanesii boiss and reut, Les Technologies de Laboratoire 5 (19) (2010) 10-15.
[6] O. Toubal, A. Djahoudi, A. Bouzabata, Preliminary studies and antimicrobial evaluation of the aerials parts of Genista numidica ssp. numidica of Genistanumidica ssp. Numidica, Journal of Life Sciences 5 (2011) 954-959.
[7] M. Maoz, I. Neeman, Antimicrobial effects of aqueous plants extracts on the fungi Microsporumcanis 2193-2199 and Trichophytonrubrum and 03 bacterial species, Letters en Applied Microbiology 26 (2002) 61-63.
[8] A. Nostro, M.P. Germano, V. D’Angelo, A. Maino, M. Caunaelli, Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity, Letters in Microbiol. Appl. 30 (5) (2000) 379-384. [9] O. Toubal, A. Djahoudi, C. Henchiri, Phytochemical
study and antimicrobial activity of Ammoidesverticillata, an Algerian endemic species, Abstract in Current Opinion in Biotechnology, 2011, p. 143.
[10] M. Felidjo, M. Bouazza, T. Ferouani, Short paper about the fliristic community and the interest of Ammoides verticillat, a medicinal plant from Mounts of Tlemcen Natural Park (Western Algeria), Geo-Eco-Trop 34 (2010) 147-154. [11] P. Quezel, S. Santa, Nouvelle flore d’Algérie et des
régions désertiques méridionales (New Flora of Algeria and Southern Desert Areas), Vol. I & II, C.N.R.S., Paris, France, 1962-1963, p. 1170. (in French)
[12] P. Belty, Jackson, W. Derek, Atlas of Microscopy Plants Culinary Herbs and Spices Press, Adivision of Pinter Public, London, 1990.
[13] J.B. Harborne, Methods of plant analysis, in: Phytochemical Methods Chapman and Hall, London, 1973, p. 132.
[14] J.A. Kiehlbauch, G.E. Hannet, M. Salfinger, W. Archinal, C. Monserrat, C. Carlyn, Use of the national committee, in: L. Duraffourd (Ed.), Traité de Phytothérapie Chimique (Chemical Treaty of Phytotherapy), Edition Masson, 1987. (in French)
[15] L. Duraffourd, Traité de Phytothérapie Chimique (Chemical Treaty of Phytotherapy), Edition Masson, 1987. (in French)
[16] F. Bssaibis, N. Gmira, M. Megrane, Activité antibactérienne de Dittrichia viscosa (L.) W. Qreuter, Microb. Indu. San. et Envir. Maroc 3 (1) (2009) 44-55. [17] J.Y. Lee, Y.S. Kim, D.H. Shin, Antimicrobial synergistic
effect of linolenic acid and monoglyceride against Bacillus cereus and Staphylococcus aureus, J. Agric. Food Chem. 50 (7) (2002) 2193-2199.
Journal of Life Sciences 6 (2012) 248-252
The Survey of Microbiological Contamination of Pitcher
Cheese in West Azarbayjan Province, Iran
Ehsan Barati1, Mona Daneshgar Moghaddam1, Navab Ghobadi2, Hamid Reza Shafieian3 and Abbas Barin4
1. Islamic Azad University, Tehran Branch, Tehran 6516935897, Iran
2. Payame Noor University, Bahar Branch, Bahar 6531855584, Iran
3. Shahid Maghsoodi, Teachers Training College, Hamedan, Iran
4. Department of Microbiology, Veterinary Medicine Faculty, Tehran University, Tehran 645314155, Iran
Received: August 11, 2011 / Accepted: September 21, 2011 / Published: March 30, 2012.
Abstract: Milk acts as a suitable peripheral culture for growth and propagation of different kinds of micro organisms. During the process of producing cheese, some micro organisms such as Escherichia coli, Coliform, Staphylococcus, Mold and Yeast may cause its contamination. In respect to the fact that pitcher cheese is produced in traditional way in different regions in West Azarbayjan, the aim of this research is examining the rate of contamination of pitcher cheese in West Azarbayjan. About 42 samples of pitcher cheese were gathered under strill condition from different parts of West Azarbayjan. In order to study microbes contamination, the samples were examined by standard microbiologic ways in laboratory from the 42 samples of pitcher cheese, four samples were contaminated by Staphylococcus aureus coagulase positive, 16 samples were contaminated by E. Coli, 21 samples by Coliform, 17 samples by mold and yeast. The producing and delivering should be controlled because of the rate of contamination in pitcher cheese and this kind of cheese should be produced in half industrial way by controlling and making special facilities for pitcher cheese producers.
Key words: Pitcher cheese, E. coli, coliform, mold and yeast, staphylococc.
1. Introduction
The pitcher cheese has the biggest consumption in rural and citizen areas of West Azarbayjan, East
Azarbayjan and more or less in other parts of Iran. It is produced from sheep milk, goat milk and sometimes from cow milk. To get the cheese with better flavor and
more stature, the cheese is produced without heating and by adding some natural starter. After clotting or
refilling, the clot is salted and laid in pitches and the openings are covered and fastened with cloths and the pitchers are laid under wet soil. The cheese is used after
six months.
This research has been done in different areas of West
Azarbayjan in order to show the rate of contamination of coliform, E. coli, Staphylococcus aureus, mold and yeast
Corresponding author: Ehsan Barati, D.V.M., research field: microbiology. E-mail: [email protected].
because of producing unstrilled cheese in rural areas of Iran and not caring for health cases.
Staphylococcus bacteria are the most important spieces of micrococcus family. It is the factor of more than 80% of pustular infections. Food poisioning from
the poision of Enterotoxin, Staphylococcus is widely spread all over the world that causes gastroenteritis and
is caused mainly because of contamination with different biotypes, Staphylococcus aureus. Human or cattle are a potential resource of milk contamination
and its products during breast pumping, producing and delivering milk products. Rate of death by this
poisoning is very rare, although 1,500 children who were poisoned by dried milk, died [1].
Forty percent of spread of gastroenteritis were
caused by food factors in Hungary. This percent is reported less in Japan (10%-25%). The cause of food
The Survey of Microbiological Contamination of Pitcher Cheese in West Azarbayjan Province, Iran
249
Britain 15% of 359 cases of food poisoning from enterotoxin staphylococcus that was reported in
1969-1990 were from red meat, poultries and their products [2]. Other cases in Britain were related to milk
contamination of the cheese which was transferred by chest swelling [3]. In Turkey the study in 50 samples of Cara cheese showed that the reason of high existence of
Staphylococcus aureus was the use of raw milk and unsuitable conditions while saving and producing the
cheese [4]. Haegbert et al. had a 2-continuous-year study (1999-2000) of poisioneal food with
Staphylococcus aureus in France, which showed that
32% of its contamination were related to milk products specially cheese [5].
E. coli is the most common aerobic organism in digestive system in men and most animals, and the existence of this organism in water and nutrients is also
the rate of contamination index. Consideration of health conditions in the process of producing and
keeping, and also the quality of raw milk is necessary. The entrance resources were the consumed water and instruments.
Turanta et al. [6] did experiments on 38 samples of white Turkish cheese and they declared the existence of
coliform in this cheese. The researches were done on Orgu cheese in Turkey by Turkoglue et al. who showed that coliform was separated from this cheese [7].
Fungi in our surroundings are microorganisms that spread widely in our surroundings and because of this
spreading we can consider them as a part of natural floor of a nutrient. In an experiment that Grasi et al., did on Manura cheese, they separated the mold and yeast from
the samples that its average was 7.41 log CFU/g [8].
2. Materials and Methods
2.1 Sampling
Forty two samples of pitcher cheese (each samples 200 g) were gathered randomly from nine areas of
West Azarbayejan in autumn-winter in 2010-2011. All the samples were transferred to the laboratory in
conical flask and under fridge conditions. The glasses
were covered with aluminium foils. Each sample with a completed form containing information about the site
of sampling was sent.
2.2 Chemical Analysis
The rate of the pH in all samples which was done
with pH meters (sartarius) was sent to the lab.
2.3 Microbial Analysis
The cheese samples were homogenized by
mechanical mixer and complete care was done for the test by sodium sitrate solution 2%.
2.4 Method of the Test
2.4.1 Coliform Culture
A plate was considered for each sample and the characteristics of the samples were written on the plate.
1 mL of the sample was transferred to the plate while caring for sterilization cases.
About 15-20 mL of the culture violet red blood agar
(VRBA), whose temperature was 45 °C, was poured
in each plate and the culture and the sample were
mixed well with a rotation movement. After it got into solid form, about 5-10 mL culture was added to it again
and it got into solid form again. The cultured plates
were kept upside down in the hot-house 31 °C for 24
hours.
2.4.2 The Confirming Test
One fifth of the suspicious colonies was chosen and
injected to the testing tube containing Dorham tube and
common BGb culture. The tubes were kept in the
hot-house 31 °C for 24 hours. The existence of gas at the end of incubation emphasized the coliform [9].
2.4.3 Method of Searching E. coli
This method showed that E. coli is based on
producing gas because of lactose fermentation and producing indole from tryptophan analysis at 44 °C.
1 mL of the confirmed positive coliform (BGb tube containing Dorham) was added to the culture Brilliant lactose Broth and another 1 mL added to peptone
The Survey of Microbiological Contamination of Pitcher Cheese in West Azarbayjan Province, Iran
250
The testing tubes were laid in the incubator 44 °C for 48 hours. At the end of the incubation period the
sample would have been positive showing E. coli if the gas had been produced in the tube containing the
Brilliant lactose Broth. Then 5 mL Coax were added to the tube containing peptone water after incubation and it was mixed well. The result was studied after one
minute. The tubes whose surfaces got red showed positive reaction of endol and the sample was positive
of E. coli [9].
2.4.4 Staphylococcus Culture
25 g of each samples of the cheese were separated in
strilled form for testing the existence of staphylococcus and the meat was added to the 70 mL and they were
laid at 37 °C for 24 hours after hemogenation. Then the samples were cultured on Barid Parker and they were incubated at 37 °C for 48 hours. Gram coloring was
done on the colonies suspicious of having staphylococcus. The catalase test would have been
done if the gram positive cocci had been observed. Coagulase test was done in the tube method for positive gram bacteria and positive catalase. The positive
coagulase bacteria were interpreted as Staphylococcus aureus [10].
2.4.5 Yeast and Mold Culture
1 mL of the samples was cultured briefly in the chosen site. The plates were laid in an aerobic
condition at 25 °C about 3-5 days. Then the mold and yeast colonies were counted separately and their rates
in gram were reported from the samples.
2.5 Statistical Analysis
Statistical computing was done by software SPSS copy 12 (SPSS Inc. and Chicago, IL, USA) by using K
square.
3. Results and Conclusions
In the studies done by Turanta et al. on 38 samples of Turkish cheese, a few staphylococcus were
separated [6]. And also Deluca et al. in Bolonia Italy and Araujo et al. in Brazil illustrated that the cheese
had staphylococcus contamination [11]. In a study that was done on 25 samples of Irish rural cheese by
Convey et al., 10 samples having positive
Staphylococcus aureus were reported [12]. And also in
a research that was done on three samples of Italian cheese by Dicargo et al., the rate of staphylococcus was estimated 4.2-4.4 log CFU/g [13].
In this study that, performing on 42 samples of pitcher cheese gathered from different parts of West
Azarbayjan four samples, showed contaminated by
Staphylococcus aureus Coagulase positive and the most contaminated sample was observed in Mahabad.
Sample number six of Bookan area had the most contamination with Staphylococcus areus (3.8 × 10
CFU/g) that was higher than the usual level. This shows the unsuitable health conditions while producing, keeping, transporting cheese by personal and if the
initial numbers of this cheese are high, even the reduction of pH in the time of clotting can’t prevent the
production of toxin and occurring staphylococcus poisoning.
In different traditional cheese, Staphylococcus
aureus disappeared while producing and it is caused because of skin, nose, mouth contamination of the
related personals. So if the heating control and health cases are not considered, contamination with staphylococcus will occur.
As a result of the experiments that were conducted on 38 samples of white Turkish cheese by Turantus et
al., the amounts of coliform and E. coli were separated from these samples [6]. Hayaloglu et al. researches on Tulum cheese showed coliform as much as 5.01 log
CFU/g [14]. In another study that was done on nine samples of Anevato cheese by Hatzikamari et al.,
contamination of this cheese with coliform is as much as 7.01 log CFU/g [15].
Convey et al. reported the contamination of most
samples with coliform as much as 10 × 10 CFU/g [12]. In a study that was performed by Deker et al. on the
The Survey of Microbiological Contamination of Pitcher Cheese in West Azarbayjan Province, Iran
251
CFU/g. In a research that was done on Fossa cheese by Gobbeti et al., the samples were negative considering
contamination with E. coli [16].
In the present study that was done on 42 samples of
pitcher cheese, 21 samples showed contamination with coliform and 16 samples showed contamination with E. coli where these contaminations were more than the
other studies that had been done on other kinds of cheese. The most contamination with coliform and E.
coli was observed in Urimia and the most rate of contamination with coliform was observed on sample number 12 in Bookan that was about 2.1 × 10 CFU/g. It
seems that the existence of coliform and E. coli in the samples is because of the percent of the added salt to
the cheese and not reducing pH in the products as well as not caring for health conditions in producing and clotting. The coliforms can be checked in order to
control intestine E. coli in the cheese because the method of producing, and clotting can be main
resource of contamination and coliform growing. During producing soft cheese and half soft cheese, reducing pH factor is the cause of controlling E. coli,
so pH will increase when the salting percent is high, therefore it must be avoided to add salt in order to
prevent E. coli growth in the cheese again. The study shows the pathogen intestine E. coli growth in the cheese having pH less than five.
In the studies that Turkuglue et al. did on Orgu Turkish cheese, the mold and yeast were separated
from all samples that the rate was about 5.5 log CFU/g
[7]. In another study that was done on Tulum cheese by Hayaloglu et al., the rate of contamination to mold and
yeast was about 5.2-6.041 log CFU/g [7].
In this study that has been done on 42 samples of
pitcher cheese, 17 samples had contamination with mold and yeast and the most contamination was observed in Urumia and the sample number 42 of
Mahabad area had the most contamination by mold and yeast (3.8 × 10 CFU/g). It seems that the reason of the
contamination of cheese with mold and yeast is the existence of surrounding contaminations while filling the pitchers, the pots related to micro organisms, don’t
care for health cases by personal and the existence of moisture in these products while keeping and clotting
of the cheese under the soil. And of course no suitable lidding over the pitchers provides enough oxygen for yeast and mold growings.
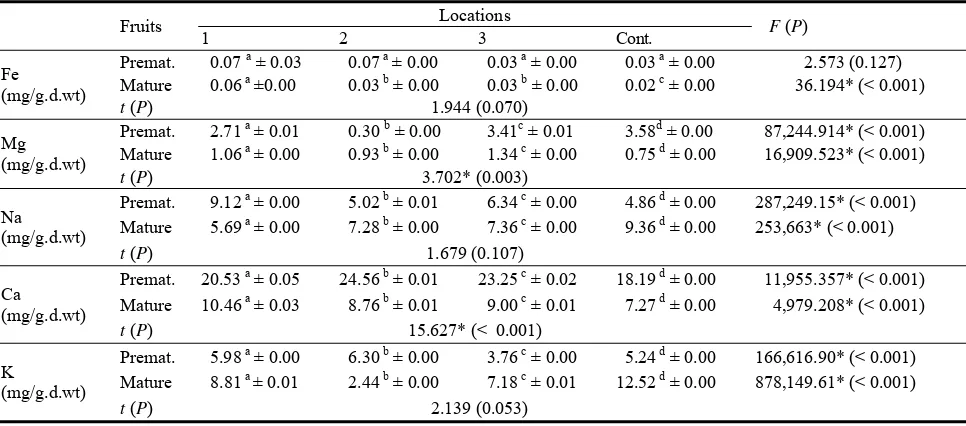
In Table 1, the spread of contamination of pitcher cheese in Azarbayjan has been shown separately based
on the place of sampling.
It must be noted that the findings were performed statistically with SPSS software copy 13, and no
meaningful difference was observed in relation to microbial contaminations of the studied areas.
The rate of pH in all samples was about 4.05-5.20 and no relation was observed between the rate of pH and got positive of the samples.
This study showed that the microbial contamination of pitcher cheese had been provided from different parts
of Azarbayjan, indicating very bad health conditions
Table 1 Spread of contamination of pitcher cheese in Azarbayjan places.
City Samples Staphylococus E. coli Coliform Mold & yeast
Bookan 15 6.6% 46.6% 66.6% 53.3%
Piranshahr 5 20% 20% 20% 20%
Khoi 2 - - - 50%
Naghadeh 3 - 33.3% 33.3% -
Makoo 5 - 20% 40% 20%
Uromia 4 - 75% 75% 75%
Salmas 4 - 25% 25% -
Mahabad 4 50% 50% 50% 75%
The Survey of Microbiological Contamination of Pitcher Cheese in West Azarbayjan Province, Iran
252
during producing, clotting, keeping transferring of
these kinds of cheese. In order to improve the microbiologic qualities of these kinds of cheese, it is
recommended to use strilled milk or enough heated milk instead of raw milk, and these kinds of cheese should be produced under good producing conditions,
good clotting and keeping, good transferring and it is recommended to study more to get more information
about the process of contaminating the cheese.
References
[1] M.S. Bergdoll, Staphylococcal intoxications, in: H. Riemann, F.L. Bryan (Eds.), Food Borne Infection and Intoxications, 2nd ed., New York, Academic Press, 1998, pp. 343-351.
[2] A.A. Wieneke, D. Roberts, J.R. Gilbert, Staphylococcal food poisoning in Kingdom, 1969-1990, Epidemiol Infect 110 (1993) 519-531.
[3] J.M. Jay, Staphylococcal gastroenteritis, in: Modern Food Microbiology, 3rd ed., New York, Van Nostrand Reinhold Company, 1986, pp. 437-458.
[4] O. Aygun, O. Aslantas, S. Oner, A survey on the microbiological quality of Carra, a traditional Turkish cheese, J. Food Engineering 66 (2005) 401-404.
[5] S. Haeghebaert, F. Le Querrec, A. Gallay, Les Toxi-infection alimentaires collective en France en 1999 et 2000, Bull. Epidemiol. Hebdo. 23 (2002) 105-109. [6] F. Turanta , A. Unluturk, D. Goktan, Microbiological and
compositional status of Turkish white cheese international, Journal of Food Micibiology 8 (1988) 19-24.
[7] H. Turkoglv, Z. Ceylan, K.S. Dajisolyv, The micribiological and chemical quality of Orgv cheese produced in Turkey, Pakistan J. of Nutrition 2 (2003) 92-94.
[8] E. Gerasi, E. Litopoulov, N. Tzanaetakis, Microbiological stody of manura, ahard cheese made from raw ovine milk in the Greek islaan sifnos, International J. of Dairy Technology 156 (2) (2003) 117-122.
[9] E.J. Baron, S.M. Finegold, Enyerobacteriaceae, in: Bailey and Scott’s Diagnostic Microbiology, 11th ed., Mosby, St. Louis, Missouri, USA, 2002, pp. 481-487.
[10] E.J. Baron, S.M. Finegold, Staphylococcus, micrococcus, and similar organisms, in: Bailey and Scott’s Diagnostic Microbiology, 11th ed., Mosby, St. Louis, Missouri, USA, 2002, pp. 218-229.
[11] J.A. Centeno, J.L. Rodrigves, A. Cepeda, Microbiological study of ARZUA cheese (NW Spain) throughout cheese Makin and Ripning, J. of Food Safety 14 (3) (2007) 229-241.
[12] M. Coveney, M. Helen, G.F. Fitzgerald, C. Daly, A study of the microbiological status of Irish farmhouse cheeses with emphasis on selected pathogenic and spoilage micro-organisms, J. Applied Bacteriology 77 (1994) 621-630.
[13] R. Dicango, J. Banks, L. Sheephan, F. Fox, E.Y. Brechany, A. Corsetti, M. Gobbetti, Comparison of the microbiological, compositional biochemical volatitle profile and sensory characteristic of three Italian PDO ewes milk cheese, International Dairy J. 13 (12) (2003) 961-972.
[14] A.A. Hayaloglu, S. Cakmakci, E.Y. Brechany, Microbiology, biochemistry, and volatile composition of tulum cheese ripend in Goat’s skin or plastic bags, J. Dairy Sei. 90 (2007) 1102-1121.
[15] M. Hatzi kamari, E. Litopovlov-tzanetaki, M. Tzunetakis, Microbilogical characteristics of Anevata: Atraditional, J. Applied Microbiology 87 (1999) 595-601.
Journal of Life Sciences 6 (2012) 253-259
Detection of “Candidatus Phytoplasma asteris” in
Brussels Sprout and Its Possible Association with
Flower Bud Failure in Poland
Maria Kamińska1, Hanna Berniak1 and Piotr Kamiński2
1. Laboratory of Virology, Department of Plant Protection, Research Institute of Horticulture, Skierniewice 96-100, Poland
2. Department of Genetics, Breeding and Biotechnology, Research Institute of Horticulture, Skierniewice 96-100, Poland
Received: October 07, 2011 / Accepted: November 16, 2011 / Published: March 30, 2012.
Abstract: Severe growth abnormalities including shoot stunting, leaf blade reduction and flower bud failure of Brussels sproutwere observed in Poland. The presence of phytoplasma in diseased as well as in healthy looking plants, was demonstrated by nested polymerase chain reaction assay employing phytoplasma universal rRNA primer pairs-P1/P7 followed by R16F2n/R16R2. Products of PCR primed by R16F2n/R16R2 primer pair from naturally infected Brussels sprouts were sequenced. Comparison of the obtained 16S rDNAs revealed high nucleotide sequence identity between analyzed phytoplasma isolates (99.8%-100%). They were also nearly identical with the sequences of other phytoplasmas isolates from sub-group 16SrI-B, and they were classified as members of “Candidatus Phytoplasma asteris”.
Key words: Brussels sprout, flower bud failure, phytoplasma, 16S rDNA.
1. Introduction
Phytoplasmas are non-culturable, wall-less prokaryotes (class Mollicutes) causing yellows and witches’ broom type diseases. The diseases have been distributed worldwide in several hundred plant species. During the past two decades, phytoplasmas have been found in many herbaceous and woody plant species with several types of symptoms using polymerase chain reaction (PCR) for detection and identification [1, 2].
In the past, numerous reports on such disorders were based merely on symptom expression and pathogen/vector relationships. Some of these disorders are now clearly assigned to identified phytoplasmas. Recently, molecular methods based on sequence and restriction fragment length polymorphism (RFLP) analysis of ribosomal genes (rDNA) specifically
Corresponding author: Maria Kamińska, Prof., research fields: plant virology, virus and phytoplasma diseases of horticulture and agriculture crops. E-mail: [email protected].
amplified by means of PCR technology, have been used. These molecular methods have been used to detect and characterize phytoplasmas in a number of plant and insect hosts.
Detection of “Candidatus Phytoplasma asteris” in Brussels Sprout and Its Possible Association with Flower Bud Failure in Poland
254
Brassica rapa) [11, 12]. In 2000, in southwestern Texas, about 5% of cabbage plants displayed symptoms of purple leaf discoloration and sprout proliferation characteristic of phytoplasma [13, 14]. Very recently in Iran, similar cabbage disease yellows, damaged cabbage up to 50% in certain fields [15].
On the basis of molecular analyses, phytoplasmas associated with shoot proliferation, flower virescence and malformation of plants belonging to the Brassica family in Europe and USA were identified as members of the “Ca. phytoplasma asteris”, subgroup 16SrI-B [6, 8, 13, 14]. Most phytoplasmas associated with yellows-type symptoms of canola plants in Canada belong to the subgroup 16SrI-A or 16SrI-B [11, 12], while phytoplasma associated with Iranian cabbage disease is related to “Ca. phytoplasma trifolii”, subgroup 16SrVI-A [15].
Brussels sprout, as well as other brassica vegetables, is an economically important vegetable crops in Poland. They are grown worldwide in cool regions of Europe and America for their content of valuable vitamins, folate, and phenolic compounds together with vitamin C are the major antioxidants in Brussels sprouts. In 2009, a new disease seriously damaged some Brussels sprout plants in Poland. The affected plants were stunted, with severely malformed leaves and flower bud failure [16]. To our knowledge, no information is available on these types of symptoms in this crop. The aim was to describe the disease symptoms and to determine whether they were associated with phytoplasma infection, and if so, to identify the pathogen. In an attempt to identify the Brussels sprout disease, standard diagnostic methods were used including PCR-RFLP and sequence analysis of phytoplasma 16S rDNA.
2. Materials and Methods
2.1 Symptom Observation and Plant Material
The observations were carried out on breeding lines of Brussels sprout plants growing in the greenhouse in the former Research Institute of Vegetable Crops, now
Institute of Horticulture, Skierniewice, Poland. Plants of four Brussels sprout lines obtained from seeds were cultivated in the field in 2008. At the end of October healthy looking plantings with desired morphological characters for the breeding purposes were taken for the generative propagation. Plantings were potted and rooted in sterile medium and grown in 15 °C - 22 °C for five weeks. All genotypes were vernalized for three months at 5 °C - 7 °C and natural photoperiod. The vernalized plants were maintained in the greenhouse at a temperature of 12 °C - 24 °C. Fertilization and pest and disease control followed the current requirements and recommendations for Brussels sprouts.
For PCR amplification, samples of leaves were collected from 12 symptomatic plants (line CAP) with stunted growth and leaf malformation and from 10 apparently healthy looking Brussels sprout plants (lines AP, P5 and DE) without disease symptoms. Samples of leaves of Catharanthus roseus inoculated by grafting with the reference strains of aster yellows phytoplasma (AY1, 16SrI-B, kindly supplied by Dr. I.M. Lee, Beltsville, USA) were also included in this study.
2.2 DNA Extraction and PCR Amplification
Total DNA was extracted from fresh leaf midribs using DNeasy Plant Mini Kit (Qiagen, Biokom, Poland) according to the manufacturer’s recommendation.
Extracted nucleic acids were used as templates for direct PCR with universal primers P1/P7 [17, 18]. Products from the first PCR were diluted 25 times and then used in nested reactions as templates for amplification with universal primers R16F2n/R16R2 [19]. All the PCR assays were run under parameters described previously [20].
Detection of “Candidatus Phytoplasma asteris” in Brussels Sprout and Its Possible Association with Flower Bud Failure in Poland
255
2.3 Sequencing and Computer Analysis
PCR amplified products (obtained with primers R16F2n/R16R2) from three samples (CAP, 218 and DE) were used for phytoplasma 16S rRNA gene sequence analysis. Sequencing was performed in an AbiPrism 3100 Genetic Analyzer apparatus (Applied Biosystems, USA), at the Maria Skłodowska Memorial Cancer Center and Institute of Oncology, Warsaw, Poland.
Aligned nucleotide (nt) sequences of 16S rRNA gene fragments were analyzed and their identities determined using the Lasergene v. 7.1 software package (DNASTAR Inc., Madison, WI., USA). The sequences were then checked for the similarity to known sequences, using the BLAST service available at http://blast.ncbi.nlm.nih.gov/Blast.cgi [21]. A phylogenetic relationship of tested isolates was estimated using the neighbour-joining method in MEGA software v. 4.0.2 [22].
3. Results
3.1 Symptoms
The affected glasshouse-grown Brussels sprouts in the second year of their growth developed conspicuous symptoms that included severely stunted growth, leaf malformation associated with severe leaf blade reduction, leaf chlorosis and flower bud failure (Fig. 1). The roots of the diseased plants were reduced and showed damage not related to fungus, bacteria or other pest infestation.
The occurrence of symptoms was noted on 25 plants of the male sterile line CAP. The most severe symptoms were observed in Brussels sprouts from the beginning of April till the middle of May. At the end of May most of the affected plants recovered and the new developed leaves showed mild malformation. Healthy looking Brussels sprouts of the other lines (P5, AP and DE) grown in the same conditions remained free of symptoms. However, in the following months some of them showed pods and seeds which were shriveled, often malformed and underweight.
Fig. 1 Severe leaf malformation of phytoplasma affected
Brussels sprout.
No disease symptoms suggesting phytoplasma infection were observed in Poland in other glasshouses or fields growing Brussels sprouts of several cultivars and lines.
3.2 Phytoplasma Detection and Identification
Specific products were obtained in direct PCR with the universal primer pair P1/P7 (expected length ~ 1.8 kb) only for the control samples of the reference strain AY1. No visible product was amplified by the direct PCR from samples obtained from Brussels sprout and healthy periwinkle plants.
However, in nested PCR with R16F2n/R16R2 primer pair a specific product (1.25 kb) was obtained for DNA samples collected from leaves of two out of four symptomatic Brussels sprout plants, and from three out of three symptomless Brussels sprout plants, all collected in the first day of April. No specific product was obtained for DNA samples collected at the end of April from leaves of eight symptomatic nor eight healthy looking Brussels sprout plants.
Detection of “Candidatus Phytoplasma asteris” in Brussels Sprout and Its Possible Association with Flower Bud Failure in Poland
256
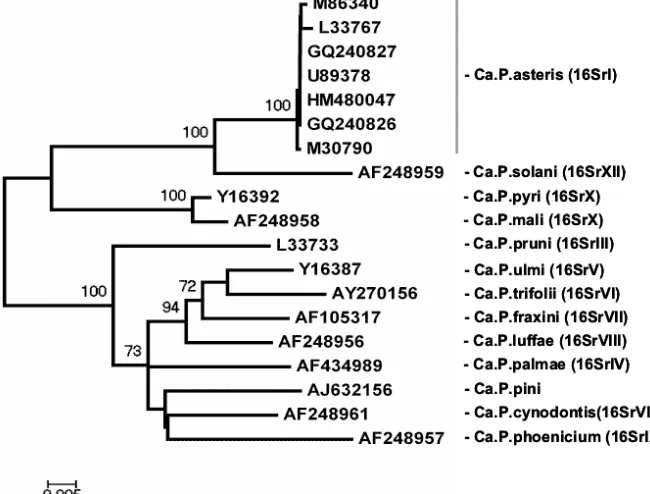
isolated phytoplasmas revealed that they were closely related to phytoplasma members of the 16SrI-B sub-group (Fig. 2). Sequences of two phytoplasmas found in B. oleracea subsp. gemmifera plants with severe leaf malformation and flower bud deficiency (line CAP) and in asymptomatic plants (line 218 and DE) (GenBank accession numbers GQ240826, GQ240827, HM480047) were identical, and they showed more than 98% identity to the sequences of ‘Candidatus Phytoplasma asteris’.
4. Discussion
The present study provides evidence for the presence of phytoplasma in Brussels sprout plants. We suggest that the observed growth abnormalities of stunted growth, severe leaf blade reduction and flower bud failure could be due to phytoplasma infection, although some of the affected plants were free of symptoms. The data obtained by sequence analysis indicated that the phytoplasma detected in Brussels sprout is nearly identical with the sequences of “Candidatus
Phytoplasma asteris”, reported from other brassicae. Our results confirm the report of Marzachi et al. [6] that this taxon, “Candidatus Phytoplasma asteris”, is implicated in the growth abnormalities in B.oleracea subsp. gemmifera plants. Based on the results of this study and other reports from Europe and North America, AY phytoplasma, mainly the subgroup 16SrI-B, seems to be the most common yellows-type disease associated with various plant species. This ranking is consistent with the top position of the AY group among other phytoplasma groups worldwide (1, 23, 24].
The disease symptoms observed in Brussels sprout in Poland are different from shoot proliferation and flower virescence reported in this crop in Italy [6] and other plants belonging to the Brassicacae family cultivated in Europe [3-5, 7] or leaf yellowing, phyllody and seed malformation observed in canola plants in Canada [12, 25], all associated with aster yellows phytoplasma infection.
The disease symptoms and the detectability of
Detection of “Candidatus Phytoplasma asteris” in Brussels Sprout and Its Possible Association with Flower Bud Failure in Poland
257
phytoplasma in Brussels sprout plants in Poland were not stable. Most of the plants with severe symptoms at the beginning of the growth season managed to partially recover in the following months and showed milder foliage malformation. The phytoplasma was detected in affected plants having 5-7 leaves but not in plants tested four weeks later. Failure to detect phytoplasma in samples of plants with severe symptoms could be due to several causes. Olivier and Galka [25] indicated that canola plants remained PCR positive only until the four-leaf stage. The results obtained suggest that the growth condition in addition to phytoplasma infection and the reaction of the host plant contribute to symptoms development and pathogen detectability. Recovery of diseased plants is a spontaneous remission of symptoms reported in grapevine, apple and apricot plants [26]. A physiological basis for this phenomenon is not yet understood. Musetti et al. [26] observed that recovery from phytoplasma-associated diseases was accompanied by an overproduction of hydrogen peroxide (H2O2), localized in the phloem tissue. We are
not able to assert whether the changes in the severity of symptoms and phytoplasma detection in affected Brussels sprout plants were related to the pathogenicity of phytoplasma, the reaction of the host plant, or growth condition.
The present study provides evidence that phytoplasma apparently occurred in such a low titre in Brussels sprout that detection by direct PCR amplification using universal primers was not possible. However, phytoplasma could be detected through nested PCR assays of the product obtained by direct PCR. This result is in agreement with the findings of Wang and Hiruki [11] and Olivier and Galka [25] who were not able to detect the aster yellows phytoplasma in canola plants by direct PCR in Canada. Contrary to those results, in Italy and Greece [6, 8, 10], Texas [14] and Iran [15], having relatively warm climate, titre of phytoplasmas in Brassica spp. plants was higher than in Poland or Canada and they were detectable by direct
PCR.
Our results that aster yellows phytoplasma was present in symptomatic as well as symptomless plants indicate that there was no close association between the presence of symptoms and the finding of positive PCR. Similar problems with phytoplasma detection in diseased plants was experienced in Canada by Olivier and Galka [25] who revealed that the percentage of phytoplasma infected B. napus and B. rapa plants is greater than the percentage of plants showing AY symptoms. It is possible that asymptomatic plants are in an incubation period or that they may be more tolerant. Symptomless infection is important in the ecology and epidemiology of phytoplasma associated diseases, because latently infected plants may serve as a source of pathogen inoculums for crops, and as survival hosts. Firrao [27] provided evidence that phytoplasma may occur in very low concentration in plant species that is not considered as hosts for phytoplasmas, and provoke mild or no symptoms. Firrao et al. [28] also conclusively demonstrated that most of the symptoms are related to the host plant rather than to the pathogen genetic background. It seems likely that the climate of Poland promote the latent infection in Brussels sprout as well as in other crops like lily [29], tulip [30], rose [31] and bleeding heart [32]. Detection of phytoplasmas in diseased as well as apparently asymptomatic Brussels sprouts underscores the need to clarify the role of phytoplasmas in the development of disease symptoms of uncertain etiology.
References
[1] E. Seemüller, C. Marcone, U. Lauer, A. Ragozzino, M. Göschl, Current status of molecular classification of the phytoplasmas, Journal of Plant Pathology 80 (1998) 3-26. [2] I.M. Lee, R.E. Davis, D.E. Gundersen-Rindal,
Phytoplasmas: Phytopathogenic mollicutes, Annual Review of Microbiology 54 (2000) 221-255.
Detection of “Candidatus Phytoplasma asteris” in Brussels Sprout and Its Possible Association with Flower Bud Failure in Poland
258
Mediterranea 29 (1990) 107-113.
[4] C. Marcone, A. Ragozzino, E. Seemüller, Detection and identification of phytoplasmas infecting vegetable, ornamental, and forage crops in southern Italy, Journal of Plant Pathology 79 (1997) 211-217.
[5] C. Marcone, A. Ragozzino, Detection of phytoplasmas in Brassica spp. in southern Italy and their characterization by RFLP analysis, Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz 102 (1995) 449-460.
[6] C. Marzachì, A. Alma, M. d’Aquilio, G. Minuto, G. Boccardo, Detection and identification of phytoplasmas infecting cultivated and wild plants in Liguria (Italian Riviera), Journal of Plant Pathology 81 (1999) 127-136 [7] M. Vibio, A. Bertaccini, I.M. Lee, R.E. Davis, M.F. Clark,
Differentiation and classification of aster yellows and related European phytoplasmas, Phytopathologia Mediterranea35 (1996) 33-42.
[8] A. Bertaccini, Z. Vorackova. M. Vibio, J. Franova, M. Navratil, J. Spak, J. Nebesarova, Comparison of phytoplasmas infecting winter oilseed rape in the Czech Republic with Italian Brassica phytoplasmas and their relationship to the aster yellows group, Plant Pathology 47 (1998) 317-324.
[9] M. Starzycki, E. Starzycka, Parents and progeny plant deformation of Brassica napus L. infected by Phytoplasma sp., Rośliny Oleiste-Oilseed Crops 21 (2000) 399-408.
[10] V.I. Maliogka, J.T. Tsialtas, A. Papantoniou, K. Efthimiou, N.I. Katis, First report of a phytoplasma associated with an oilseed rape disease in Greece, Plant Pathology 58 (2009) 792.
[11] K. Wang, C. Hiruki, Molecular characterization and classification of phytoplasmas associated with canola yellows and a new phytoplasma strain associated with dandelions, Plant Disease 85 (2001) 546-552.
[12] C.Y. Olivier, G. Séguin-Swartz, T. Barasubiye, D. Hegedus, First report of “Candidatus Phytoplasma asteris”-related strains in Brassica rapa in Saskatchewan, Canada, Plant Disease 90 (2006) 832.
[13] I.M. Lee, R.A. Dane, M.C. Black, N. Troxclair, First report of an aster yellows phytoplasma associated with cabbage in southern Texas, Plant Disease 85 (2001) 447. [14] I.M. Lee, M. Martini, K.D. Bottner, R.A. Dane, M.C.
Black, N. Troxclair, Ecological implications from a molecular analysis of phytoplasmas involved in an aster yellows epidemic in various crops in Texas, Phytopathology 93 (2003) 1368-1377.
[15] M. Salehi, K. Izadpanah, M. Siampour, Characterization of a phytoplasma associated with cabbage yellows in Iran, Plant Disease 91 (2007) 625-630.
[16] P. Kamiński, H. Berniak, M. Kamińska, ‘Candidatus Phytoplasma asteris’ identified in Brussels sprouts and its
possible association with flower bud failure in Poland, in: 17th Crucifer Genetics Workshop BRASSICA, Saskatoon, Canada, Book of Abstracts, 2010, p. 97.
[17] S. Deng, C. Hiruki, Amplification of 16S rRNA genes from culturable and nonculturable mollicutes, Journal of Microbiological Methods 14 (1991) 53-61.
[18] B. Schneider, E. Seemüller, C.D. Smart, B.C. Kirkpatrick, Phylogenetic classification of plant pathogenic mycoplasma-like organisms or phytoplasmas, in: S. Razin (Ed.), Molecular and Diagnostic Procedures in Mycoplasmology, Academic, San Diego, 1995, pp. 369-380.
[19] I.M. Lee, D.E. Gundersen-Rindal, R.E. Davis, I.M. Bartoszyk, Revised classification scheme of phytoplasmas based on RFLP analyses of 16S rRNA and ribosomal protein gene sequences, International Journal of Systematic Bacteriology 48 (1998) 1153-1169.
[20] H. Śliwa, M. Kamińska, S. Korszun, P. Adler, Detection of ‘Candidatus Phytoplasma pini’ in Pinus sylvestris trees in Poland, Journal of Phytopathology156 (2008) 88-92. [21] S.F. Altschul, T.L. Maden, A.A. Schaffer, J. Zhang, Z.
Zhang, W. Miller, Gapped BLAST and PSI-BLAST: A new generation of protein database search programs, Nucleic Acids Research 25 (1997) 3389-3402.
[22] K. Tamura, J. Dudley, M. Nei, S. Kumar, MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0, Molecular Biology and Evolution 24 (2007) 1596-1599.
[23] C.F. Marcone, I.M. Lee, R.E. Davis, A. Ragozzino, E. Seemüller, Classification of aster yellows group phytoplasma based on combined analyses of rRNA and tuf gene sequences, International Journal of Systematic and Evolutionary Microbiology 50 (2000) 1703-1713. [24] I.M. Lee, D.E. Gundersen-Rindal, R.E. Davis, K.D.
Bottner, C. Marcone, E. Seemüller, “Candidatus Phytoplasma asteris”, a novel phytoplasma taxon associated with aster yellows and related diseases, International Journal of Systematic and Evolutionary54
(2004) 1037-1048.
[25] C.Y. Olivier, B. Galka, Consequences of phytoplasma infection on canola crop production in the Canada prairies, in: ENDURE International Conference, Diversifying Crop Protection, La grande-Motte, France, Book of Abstracts, Oct. 12-15, 2008, pp. 1-4.
[26] R. Musetti, R. Marabottini, M. Badiani, M. Martini, L. Sanits di Toppi, S. Borselli, et al., On the role of H2O2 in
recovery of grapevine (Vitis vinifera, cv. Prosecco) from Flavescence dorée disease, Functional Plant Biology 34 (2007) 750-758.
Detection of “Candidatus Phytoplasma asteris” in Brussels Sprout and Its Possible Association with Flower Bud Failure in Poland
259
Bacteria, 2001, pp. 100-102.
[28] G. Firrao, E. Gabbi, M. Dazzan, Differences in symptoms expression and pathogen concentration in various Arabidopsis thaliana ecotypes infected by the Italian clover phyllody phytoplasma, in: 13th Intern. Congress of IOM, Fukuoka, Japan, July 14-19, 2000, p. 155. [29] A. Bertaccini, M. Kamińska, S. Botti, M. Martini,
Molecular evidence for mixed phytoplasma infection in lily plants, in: J. Hammond (Ed.), Proc. 10th Intern. Symposium of Virus Diseases Ornamentals, Acta Horticulturae(ISHS) 568 (2002) 35-41.
[30] M. Kamińska, H. Śliwa, Molecular evidence for phytoplasma infection in tulip plants, Phytopathologia Polonica 24 (2002) 47-56.
[31] M. Kamińska, H. Śliwa, T. Malinowski, Cz. Skrzypczak, The association of aster yellows phytoplasma with rose dieback disease in Poland, Journal of Phytopathology 151 (2003) 469-476.
Journal of Life Sciences 6 (2012) 260-267
The Role of Heat Shock Protein 70, IgE and MMP-9
in Detecting Early Minor Myocardial Damage and
Evaluating the Efficacy of Coronary Artery Bypass
Grafting (CABG)
Amal A. Baalash1, Hala E. Hamouda1, Ghada M. Ismail2, Ibrahim K. Yassein3 and Bedir M. Ibrahim3
1. Department of Medical Biochemistry, Faculty of Medicine, Tanta University, Tanta 31111, Egypt
2. Department of Physiology, Faculty of Medicine, Tanta University, Tanta 31111, Egypt
3. Department of Cardiothoracic Surgery, Faculty of Medicine, Tanta University, Tanta 31111, Egypt
Received: October 10, 2011 / Accepted: December 12, 2011 / Published: March 30, 2012.
Abstract: The objective of this research was to identify levels of heat shock protein 70 (Hsp 70), total immunoglobulin E (IgE) and matrix metalloproteinase-9 (MMP-9) before and after coronary artery bypass grafting (CABG) surgery. Hsp 70, IgE, MMP-9, creatine phosphokinase-MB (CPK-MB), and lactate dehydrogenase (LDH) levels were measured in normal subjects (n = 20), and in patients with chronic stable angina pectoris who were referred for elective CABG, before and after performing CABG-surgery (n = 20). Compared with normal subjects, increased Hsp 70 and IgE levels, unchanged MMP-9 level, and activities of CPK-MB and LDH were found in the pre-operative patient group. Hsp 70 and IgE levels in the post-operative period were significantly reduced when compared to pre-operative period. Hsp 70 and IgE might be used as markers for detection of early minor myocardial damage, and coronary insufficiency with less overt damage than myocardial infarction, as significant changes in their levels appear before occurrence of in any changes in the levels of MMP-9, CPK-MB and LDH. Besides, Hsp 70, and IgE returning to the normal levels after CABG surgery, suggest that they could be helpful to evaluate the effect of CABG surgery.
Key words: Heat shock protein, IgE, metaloproteinases, MMP-9, myocardial ischemia, coronary artery bypasses grafting.
1. Introduction
The clinical feature of myocardial ischemia correlates with a particular biochemical pattern of inflammatory system activation. Experimental models of ischemic myocardial injury indicate that the inflammatory response after the ischemic event contributes to tissue damage [1]. So, inflammation is becoming an intriguing focus of research as a possible pathogenetic component and therapeutic target in ischemic heart disease.
Elevated values of circulating inflammatory markers,
Corresponding author: Amal A. Baalash, Ph.D., MD, associate professor, research fields: medical biochemistry and biomarkers. E-mail: [email protected].
such as C reactive protein (CRP), serum amyloid A protein, interleukin-6, TNF and interleukin-1 receptor antagonist, are commonly found in acute coronary syndrome (ACS). It has been suggested that this systemic inflammatory response may be the result of the myocardial microinfarction known to occur in that case [2].